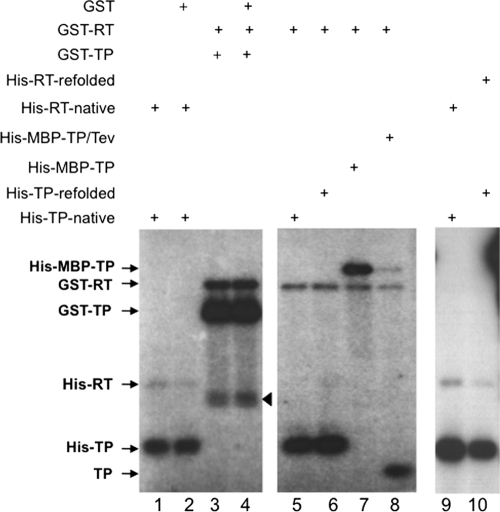
Fig. 3.
In vitro protein priming by trans-complementation of purified RT and TP domains. Purified TP and RT domains were mixed together to reconstitute protein priming by trans-complementation, in the presence of [α-32P]dGTP. The domains used were natively purified (lanes 1, 2, 5, and 9) or refolded (lanes 6 and 10) His-tagged, GST-tagged (lanes 3 and 4), His- and MBP-double tagged (lane 7), or untagged (Tev cleaved, lane 8) TP domains and natively purified (lanes 1, 2, and 9) or refolded (lane 10) His-tagged or GST-tagged (lanes 3 to 8) RT domains. GST (1 μg) was also added to the reactions shown in lanes 2 and 4. The 32P-labeled TP and RT domains (arrows) as a result of protein priming were resolved by SDS-PAGE and detected by autoradiography. The arrowhead denotes degradation products from GST-TP and/or GST-RT.