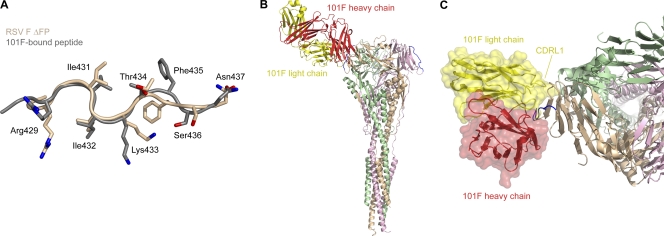
Fig. 4.
Structural conservation of the 101F epitope. The 101F epitope on the postfusion RSV F ΔFP glycoprotein is in a conformation similar to that of the 101F-bound peptide, and modeling suggests that CDRL1 contacts regions on the F glycoprotein outside the linear epitope. (A) Least-squares superposition of residues 429 to 437 from RSV F ΔFP (tan) and the 101F-bound peptide structure (gray) (28) (PDB ID 3O45), as viewed by the antibody. Side chains of residues that contact 101F in the peptide-bound structure are shown as sticks. Oxygen atoms are colored red; nitrogen atoms are colored blue. (B) Model of 101F bound to RSV F ΔFP based on the alignment in panel A. The 101F heavy chain is colored red, and the light chain is colored yellow. RSV F ΔFP residues 429 to 437 are colored blue. (C) Top view of 101F binding to RSV F ΔFP. A transparent molecular surface of 101F is shown over a ribbon representation of the heavy and light chains. The 101F CDRL1 is labeled.