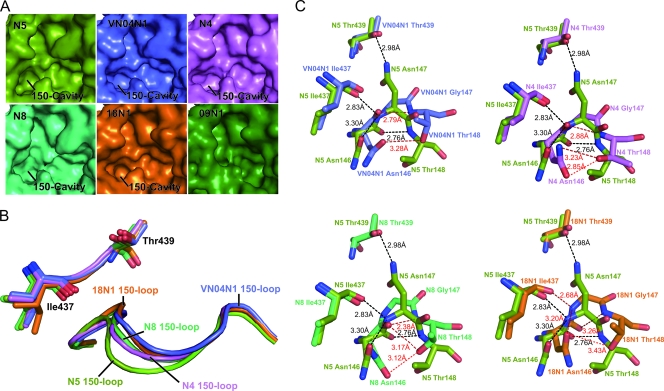
Fig. 3.
Comparison of the unique conformation of the N5 (splitpea) 150-cavity and 150-loop to those of VN04N1 (tv_blue), N4 (violet), N8 (lime green), and 18N1 (orange). (A) Comparison of molecular surfaces from all currently available group 1 NA structures, in which N5 displays an extended 150-cavity relative to those of other known structure group 1 members. (B) Comparison of N5's 150-loop with those of N4, N8, 04VN1, and 18N1. The N5 150-loop adopts an extended conformation relative to those of 04VN1, N4, N8, and 18N1. (C) Comparison of detailed polar interactions of residues 146, 147, and 148 of N5 with those of N4, N8, 04VN1, and 18N1. N5 hydrogen bonds are colored black, and N4, N8, 04VN1, and 18N1 hydrogen bonds are colored red. The upper left panel displays superimposition of residues 146 to 148 from N5 and VN04N1, the upper right displays superimposition of residues 146 to 148 from N5 and N4, the lower left displays superimposition of residues 146 to 148 from N5 and N8, and the lower right displays superimposition of residues 146 to 148 from N5 and 18N1. The presence of Asn147 in N5 promotes a further distance from Thr439, which is a key factor in the extension of the N5 150-loop relative to those in VN04N1, N4, N8, and 18N1. The distance between the Asn146 and Thr148 side chains is also further for N5 than it is for VN04N1, N4, N8, and 18N1. Furthermore, the Asn146-Asn147 peptide bond in N5 and 18N1 is rotated relative to those of VN04N1, N4, and N8, which creates an altered hydrogen bond network.