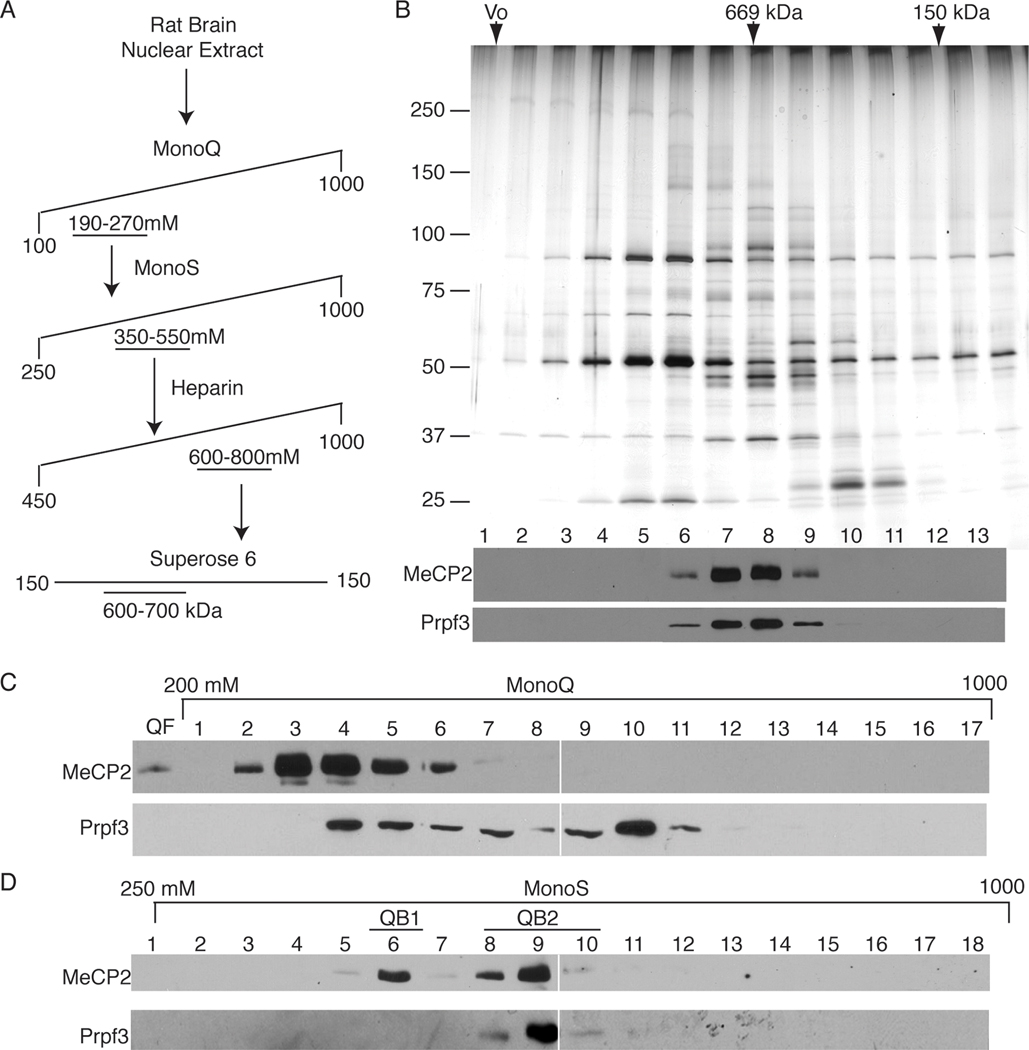
Figure 2. The QB2 pool of MeCP2 co-purifies with 6 candidate proteins including Prpf3.
(A) MeCP2 peak fractions were purified using a four-step process including the MonoQ strong anion exchange resin, MonoS strong cation exchange resin, Heparin affinity resin and by Superose6 gel filtration. (B) (Upper panel) Silver-stain analysis of the Superose6 fractionation of the QB2 MeCP2 pool. (Lower panel) Western blot analysis of Superose6 fractions shows MeCP2 protein peaks in fractions 7 and 8, precisely co-fractionating with Prpf3 protein. (C) The MonoQ fractionation of brain-derived nuclear extract reveals an overlap of Prpf3 and MeCP2 yet pools of each protein do not co-fractionate and remain independent of the other. (D) Peak fractions of the QB1/2 fractionated over the MonoS resin show two distinct pools of MeCP2 peaking at 400 mM NaCl (QB1) and 550 mM NaCl (QB2) by western blot analysis. Corresponding MonoS fractions probed for Prpf3 protein shows precise co-fractionation with QB2 Mecp2.