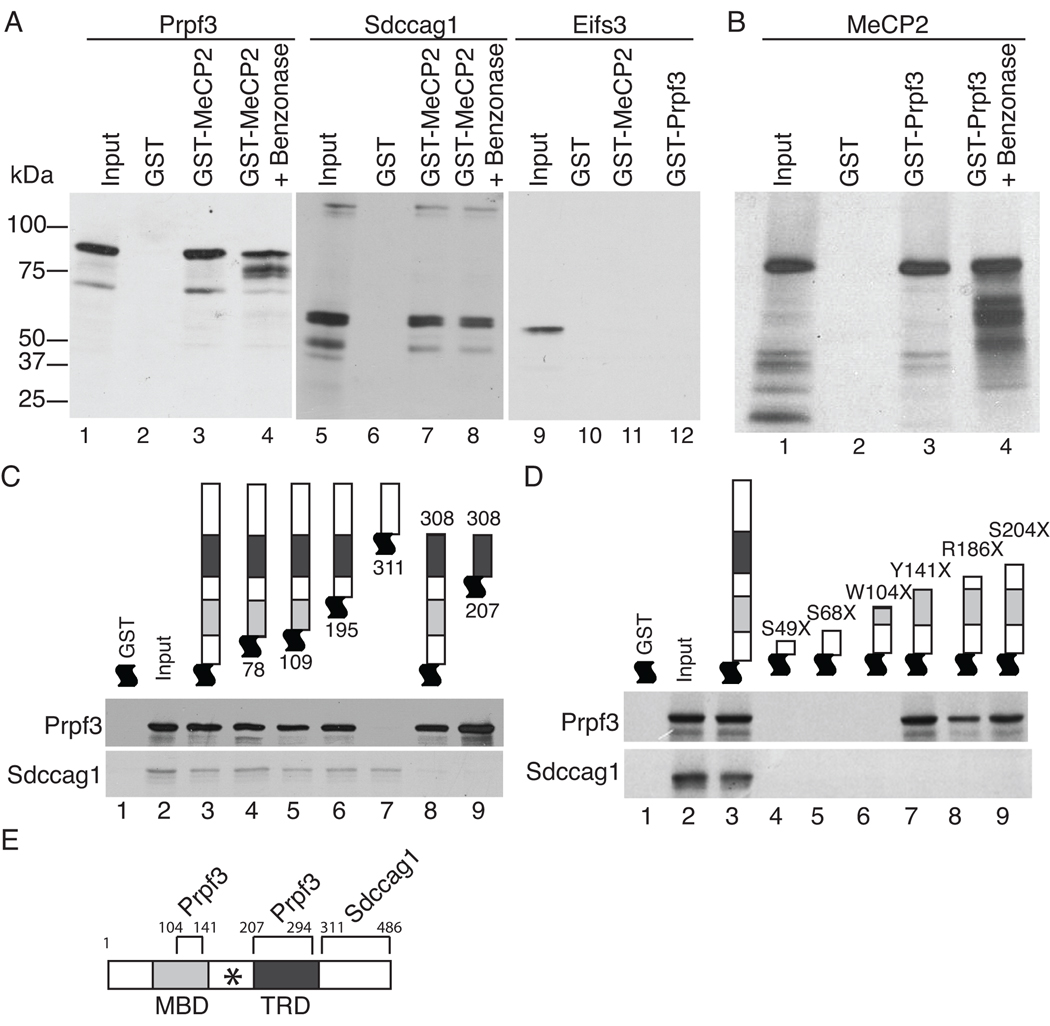
Figure 5. MeCP2 directly interacts with Prpf3 and Sdccag1.
For all experiments, the GST-tagged protein was bacterially generated and purified and the visualized [35S]-methionine labeled interacting proteins were generated by in vitro transcription and translation. Coomassie blue staining of the gels showing input GST fusion proteins are shown in Figure S4. (A) Prpf3 (left) and Sdccag1 (middle) interact directly with GST tagged MeCP2 but not GST alone. Eif2s3 (right) does not interact with either GST MeCP2 or GST Prpf3. Benzonase treatment indicates these interactions are independent of nucleic acids. (B) Reciprocally, MeCP2 interacts with GST tagged Prpf3 independent of nucleic acids. (C) GST-MeCP2 deletion constructs mapped the region of MeCP2 required for the direct interactions with Prpf3 or Sdccag1 in vitro. (D) GST tagged MeCP2 containing the indicated RTT nonsense mutations disrupt Prpf3 binding if truncations are prior to amino acid 104, but identify a second Prpf3 binding site on MeCP2 in the MBD between amino acids 104 and 141. All RTT truncations tested abolished Sdccag1 binding to MeCP2. (E) Map of MeCP2e2 protein showing the Prpf3 and Sdccag1 interaction domains with boundary amino acid numbers. The MBD, TRD, and (*) RNA binding domain [37] are indicated.