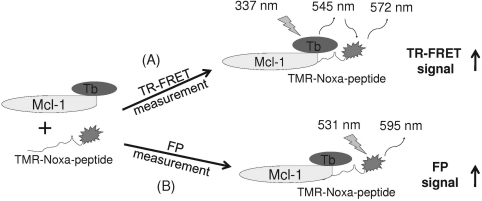
Fig. 1.
Schematic diagram of the design for the dual-readout F2 assay technology for monitoring bimolecular interactions with the Mcl-1 protein and Noxa peptide interaction as a model system. Mcl-1 protein is labeled with a FRET donor terbium, whereas its binding partner, Noxa peptide, is labeled with TMR. Binding of Mcl-1 protein to TMR-Noxa peptide brings donor (terbium) and acceptor (TMR) fluorophores together, leading to the energy transfer from terbium to TMR upon excitation at 337 nm and generating TR-FRET signal (A). Meanwhile, binding of Mcl-1 protein to TMR-Noxa peptide slows down the movement of the TMR-Noxa peptide in the same reaction, leading to the generation of FP signal (B). FP, fluorescence polarization; FRET, fluorescence resonance energy transfer; TMR, 5/6-carboxytetramethyl-rhodamine; TR-FRET, time-resolved fluorescence resonance energy transfer.