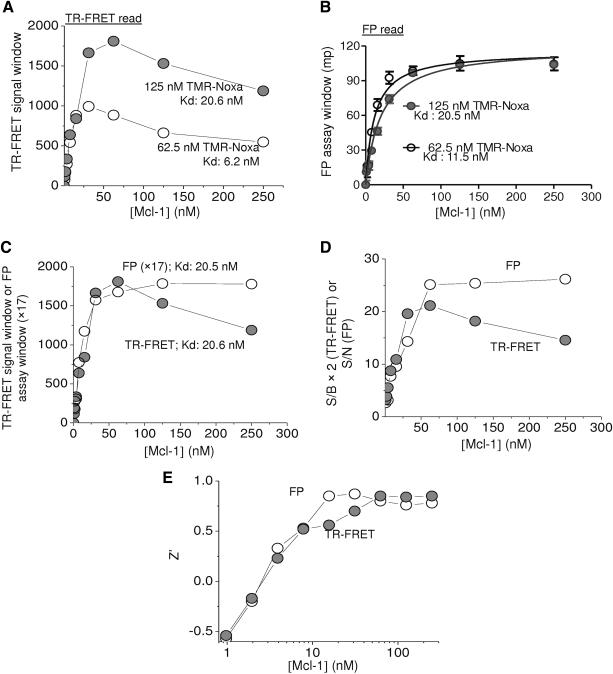
Fig. 3.
Optimization of Mcl-1 protein in dual-readout F2 assay. (A) Increasing concentrations of Mcl-1 protein were incubated with 62.5 or 125 nM of TMR-Noxa peptide and incubated at room temperature for 1 h. The TR-FRET signal and the FP signal were measured using Envision Multilabel plate reader. (A) The FRET signal window was obtained by subtracting peptide-only values from values in the presence of the amount of added Mcl-1 protein and plotted against Mcl-1 protein concentration. (B) The FP assay window was obtained by subtracting the mP values of tracer alone from the values in the presence of protein. Data were analyzed using a nonlinear regression method in Prism 5.0. (C) The comparison between dose–response curves of the binding of 125 nM TMR-Noxa peptide to Mcl-1 protein between TR-FRET and FP measurements. The values of FP assay window in mP were multiple by 17 to bring to the similar scale of TR-FRET signal window for comparison. The data shown are average with SD from four replicates. (D) The S/B values of TR-FRET (values × 2 to approximate same scale) and S/N values of FP were obtained from data in (A) and (B) for the binding of 125 nM TMR-Noxa peptide to Mcl-1. (E) Z′ values for TR-FRET and FP measurements were calculated from data in (A) and (B) for the binding of 125 nM TMR-Noxa peptide to increasing concentrations of Mcl-1 protein.