Table 3.
Scope and Selectivity in Nickel-Catalyzed Coupling of α-Olefins and Isocyanates.a
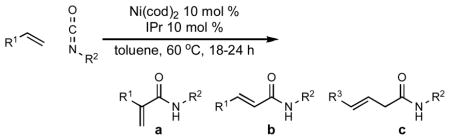 | |||||
|---|---|---|---|---|---|
| entry | R1 | R2 | R3 | product(s) | yield a; b; c (%)b |
| 1 | n-hexyl | Cy | - | 1a, 1b | 79; 14; 0 |
| 2 | n-hexyl | t-Bu | - | 2a, 2b | 74; 17; 0 |
| 3c |
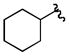
|
Cy | - | 3a | 72; 0; 0 |
| 4 |
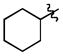
|
t-Bu | - | 4a | 91; 0; 0 |
| 5c |
|
Cy | - | 5a, 5b | 74; 5; 0 |
| 6 |
|
t-Bu | - | 6a, 6b | 71; 10; 0 |
| 7c | PhCH2 | Cy | Ph | 7a, 7b, 7c | 65; 8; 22 |
| 8c | PhCH2 | t-Bu | Ph | 8a, 8b, 8c | 83; 1d; 5d |
| 9c |
|
Cy | - | 9a | 86; 0; 0 |
| 10 |
|
t-Bu | - | 10a | 82; 0; 0 |
| 11 |
|
Cy | - | 11a, 11b | 74; 13; 0 |
| 12 |
|
t-Bu | - | 12a, 12b | 72; 12; 0 |
| 13 |
|
Cy | - | 13a | 24; 0; 0 |
| 14 |
|
t-Bu | - | 14a, 14b | 70; 2; 0 |
| 15 |
|
Cy | - | 15a, 15b | 68; 17; 0 |
| 16 |
|
t-Bu | - | 16a, 16b | 65; 9; 0 |
Standard conditions (see Supporting Information): Reactions were run with 0.5 mmol 1-octene, 1.0 mmol tert-butyl isocyanate, 0.05 mmol
Ni(cod)2, and 0.05 mmol IPr in 0.5 mL toluene under Ar(g) in a sealed
tube at 60 °C for 18–24 h.
Isolated yields.
Reaction was run using 2 mL toluene.
Isolated as a mixture of 8b and 8c, with relative ratios determined by 1H NMR.
