Table 1.
Influence of Reaction Parameters on the Ni-Catalyzed Reductive Coupling of an Alkyne and Epoxide
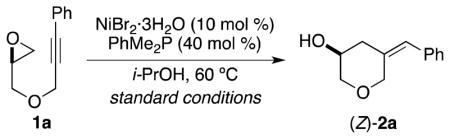 | |||
|---|---|---|---|
| entry | variation from standard conditions | yield (%)a | Z/Eb |
| 1 | nonec | 82 | 90:10 |
| 2 | Ni(cod)2 instead of NiBr233H2Od | 82 | 95:5 |
| 3 | NiBr23diglyme instead of NiBr233H2O | 76 | 88:12 |
| 4 | NiCl236H2O instead of NiBr233H2O | 74 | 89:11 |
| 5 | 5 mol % NiBr233H2O and 20 mol % | 75 | 95:5 |
| PhMe2P instead of standard catalyst/phosphine loading | |||
| 7 | THF instead of i-PrOH | <5e | --- |
| 8 | no NiBr233H2O | <5e | --- |
| 9 | 30 mol % PhMe2P instead of 40 mol % | 76 | 90:10 |
| 10 | 20 mol % PhMe2P instead of 40 mol % | 52 | 91:9 |
| 11 | no PhMe2P | <5e | --- |
Isolated yield of the mixture of olefin isomers.
Determined by 1H NMR spectroscopy of the crude reaction mixture.
Complete conversion of starting material observed within 3 h.
Phosphine loading was 20 mol % instead of 40 mol %.
Determined by 1H NMR spectroscopy relative to mesitylene as an internal standard.
