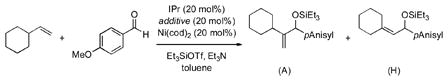
Table 1.
Evaluation of additives in Ni–IPr-mediated coupling reactions
| ||||||
|---|---|---|---|---|---|---|
| entry[a] | additive | conversion [%][b] | yield A [%][b,c] | yield H [%][b] | ||
| 1 | none | 55 | 18 (33) | 0 | ||
| 2 | mCF3-styrene | 79 | 39 (49) | 0 | ||
| 3 | CyPPh2 | 61 | 19 (31) | 0 | ||
| 4 | PPh3 | 100 | 34 (34) | 44 | ||
| 5 | EtOPPh3 | 100 | 34 (34) | 37 | ||
| 6 | (EtO)2PPh | 84 | 30 (35) | 29 | ||
| 7 | P(OBu)3 | 68 | 32 (47) | 9 | ||
| 8 | P(OPh)3 | 59 | 45 (76) | 0 | ||
| 9 | P(OPh)3 (no IPr) | 7 | 0 (0) | 0 | ||
See Supporting Information for details.
Determined by 1H NMR (amount of remaining aldehyde relative to an external standard (CH3NO2)).
Values in parentheses are yields based on conversion of aldehyde.