Table 2.
IPr–Ni–P(OPh)3-catalyzed alkene-aldehyde coupling reactions
| entry[a] | alkene (R1) | product | conversion[%][b] | yield[%][b,c] |
|---|---|---|---|---|
| 1 | nHex |
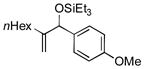
|
95[d] | 96 |
| 2 | nHex |
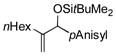
|
92[d,e] | 82 |
| 3 | nHex |
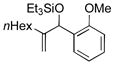
|
80 | 75 |
| 4 | nHex |
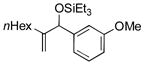
|
86 | 98 |
| 5 | Ph |
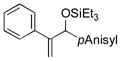
|
69 | 84 |
| 6[d] | PhCH2 |
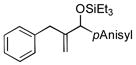
|
75 | 99 |
| 7 | PhCH2CH2 |
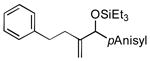
|
88 | 99 |
| 8 | Cy |
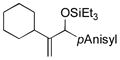
|
100 | 96 |
| 9[f,g] | iPr |
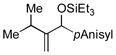
|
100 | 93 |
| 10[g,h] | tBu |
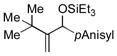
|
32 | 41 |
| 11 | iBu |
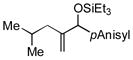
|
100 | 99 |
| 12 |
|
|
100 | 94 |
| 13 | nHex |
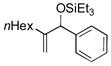
|
95 | 96 |
| 14 | nHex |
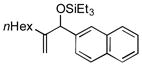
|
74 | 99 |
| 15[h] | nHex |
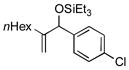
|
88 | 73 |
| 16 | Cy |
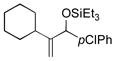
|
66 | 32 |
| 17[h,i] | nHex |
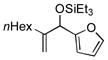
|
100 | 36 |
See Experimental Section and Supporting Information for details.
Determined by integration (1H NMR) relative to an external standard (CH3NO2).
Based on conversion.
150 mol% of alkene used.
t-BuMe2SiOTf used in place of Et3SiOTf.
1 mL of alkene used.
Reaction carried out in a sealed tube.
40 mol% of catalyst used.
Reaction carried out at room temperature.
