Table 1.
Nickel-catalyzed coupling of ethylene, aldehydes, and silyl triflates.
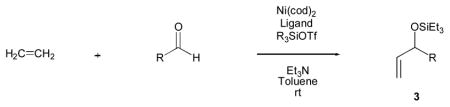 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| entry | R (aldehyde) | R3SiOTf | product | isolated yield (%) | entry | R (aldehyde) | R3SiOTf | product | isolated yield (%) |
| 1 | Ph | Et3SiOTf |
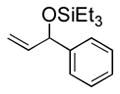 3a |
82 (65) b | 8 |
|
Et3SiOTf |
 3h |
80 |
| 2 | p-tolyl | Et3SiOTf |
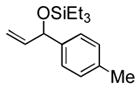 3b |
88 (65) b | 9 | 2-furyl | Et3SiOTf |
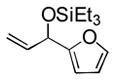 3i |
38 |
| 3 | o-tolyl | Et3SiOTf |
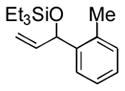 3c |
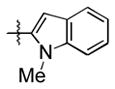
93 (64) b | 10 f |
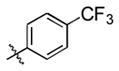
|
Et3SiOTf |
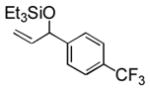 3j |
25 |
| 4 | p-anisyl | Et3SiOTf |
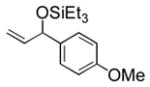 3d |
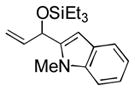
95 (65) b | 11 f |
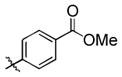
|
Et3SiOTf |
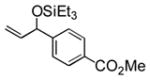 3k |
34 |
| 5 | 2-naphthyl | Et3SiOTf |
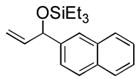 3e |
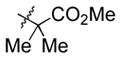
95 (83) b | 12 | piv | Et3SiOTf |
 3l |
70 |
| 6 | 2-naphthyl | Me3SiOTf |
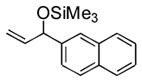 3f |
60 | 13 |
|
Et3SiOTf |
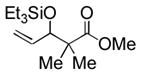 3m |
81 (40) c, d |
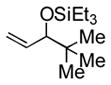
| 7 | 2-naphthyl | t-BuMe2SiOTf |
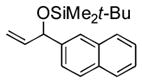 3g |
67 | 14 | cyclohexyl | Et3SiOTf |
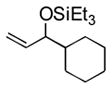 3n |
25 d (34) d,e |
Standard procedure: Ni(cod)2 (20 mol %) and (o-anisyl)3P (40 mol %) were dissolved in 2.5 mL toluene under argon. Ethylene (balloon, 1 atm) was substituted for argon. Triethylamine (600 mol %), the aldehyde (100 mol %, 0.5 mmol), and silyl triflate (175 mol %) were added. The reaction was stirred for 6–18 h at 23 ºC.
(o-anisyl)3P was replaced by Cy2PhP.
(o-anisyl)3P was replaced by Ph3P.
Yield determined by 1H NMR using DMF as a standard.
Conducted under 2 atm of ethylene.
Stirred at room temperature for 30 h.
