Table 11.
Coupling of Nitrogen-Containing Alkenes with Aldehydes a
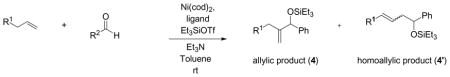 | |||||||
|---|---|---|---|---|---|---|---|
| entry | R1 (alkene) | R2 (aldehyde) | ligand | major product | yield (%) b | ratio (4:4′) b | ratio (E:Z) c |
| 1 |
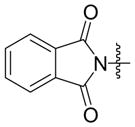
|
Ph | Cy2PhP |
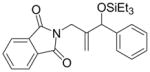 4a |
67 | 74:26 | - |
| (EtO)Ph2P |
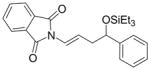 4a′ |
43 | 12:88 | 60:40 | |||
| 2 |
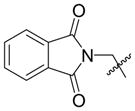
|
o-anisyl | Cy2PhP |
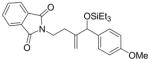 4b |
54 | 71:29 | - |
| Ph3P |
4b′ |
76 | <5:95 | 83:17 | |||
| 3 |
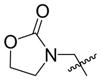
|
Ph | Cy2PhP |
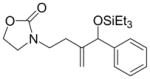 4c |
60 | 83:17 | - |
| (EtO)Ph2P |
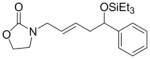 4c′ |
28 | 10:90 | n.d. | |||
Standard procedure: To a solution of Ni(cod)2 (0.1 mmol) and ligand (0.2 mmol) in toluene (2.5 mL) at 23 °C under Ar were added the alkene (1.5 mmol), triethylamine (3.0 mmol), the aldehyde (0.5 mmol), and Et3SiOTf (0.875 mmol). The mixture was stirred 48 h at room temperature and purified by chromatography (SiO2).
Determined by 1H NMR of the crude reaction mixture using DMF as a standard.
The ratio was determined by 1H NMR of the mixture of E and Z homoallylic alcohols after the silyl group of the coupling product was removed by TBAF.
