Table 4.
Effect of Bases in the Ethylene–Benzaldehyde Coupling a
 | ||
|---|---|---|
| entry | base | yield b |
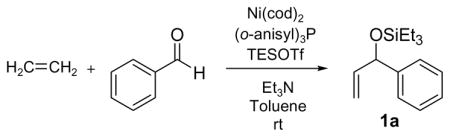
| 1 | Et3N | 77 |
| 2 | Et2NH | 3 |
| 3 | N-methylpyrrolidine | 36 |
| 4 | proton sponge | 10 |
| 5 | pyridine | 12 |
| 6 c | K3PO4 | <5 |
| 7 | K3CO3 | <5 |
| 8 | Cs2CO3 | <5 |
Standard procedure: Ni(cod)2 (20 mol%) and (o-anisyl)3P (40 mol%) were dissolved in 2.5 mL toluene under argon. Ethylene (balloon, 1atm) was substituted for argon. A base (600 mol%), benzaldehyde (100 mol%, 0.5 mmol), and Et3SiOTf (175 mol%) were added. The reaction mixture was stirred 18 h at 23 °C.
Yields were determined by 1H NMR using DMF as a standard.
Benzaldehyde was replaced by 2-naphthaldehyde and the reaction was run at 0.25 mmol scale.
