Table 9.
Preparation of Homoallylic Alcohol Products from Nickel-Catalyzed Alkene–Aldehyde Couplings a
| entry | alkene | aldehyde | major product (2) | yield (%)(2:2′) b,c | E:Z (2) b |
|---|---|---|---|---|---|
| 1 d |
|
PhCHO |
2a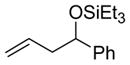
|
73 (89:11) | n.a |
| 2 |
|
PhCHO |
2b |
85 (95:5) | 75:25 |
| 3 e | 72 (>95:5) | 75:25 | |||
| 4 e | p-anisaldehyde |
2c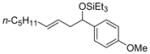
|
85 (>95:5) | 75:25 | |
| 5 e | p- Cl(C6H4)CHO |
2d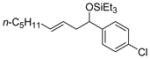
|
37 (>95:5) | 74:26 | |
| 6 f | 2-naphthaldehyde |
2e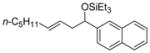
|
88 (>95:5) | 70:30 | |
| 7 | 1-methyl-2-indole- carboxaldehyde |
2f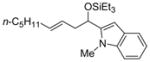
|
56 (>95:5) | 83:17 | |
| 8 f | t-BuCHO |
2g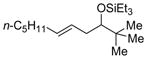
|
64 (>95:5) | 78:22 | |
| 9 |
|
PhCHO |
2h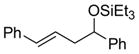
|
86 (92:8) | >95:5 |
| 10 |
o-anisaldehyde m-anisaldehyde p-anisaldehyde |
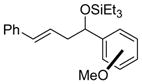 2i (ortho) 2i (meta) 2i (para) |
78 (92:8) 98 (92:8) 99 (92:8) |
>95:5 >95:5 >95:5 |
|
| 11 f,g | p-anisaldehyde | 2i (para) | 98 (92:8) | >95:5 | |
| 12 f | 2-naphthaldehyde |
2j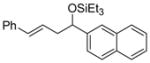
|
88 (95:5) | >95:5 | |
| 13 | 1-methyl-2-indole-carboxaldehyde |
2k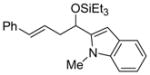
|
57 (>95:5) | >95:5 | |
| 14 | t-BuCHO |
2l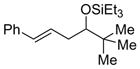
|
65 (>95:5) | 78:22 | |
| 15 |
|
p-anisaldehyde |
2m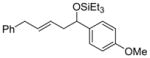
|
91 (92:8) | 69:31 |
| 16 |
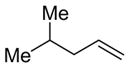
|
PhCHO |
2n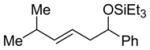
|
82 (>95:5) | 81:19 |
|
| |||||
| 17 |
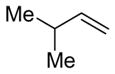
|
2o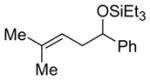
|
95 (86:14) h | n.a. | |
| 18 |
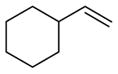
|
2p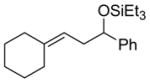
|
99 (75:25) h | n.a. | |
| 19 |
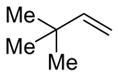
|
3q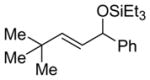
|
14 (>95:5) i | n.a. | |
Standard procedure: (entries 1–8, 15–18): To a solution of Ni(cod)2 (0.1 mmol) and EtOPPh2 (0.2 mmol) in toluene (2.5 mL) at 23 °C under Ar were added the alkene (0.5 mL), triethylamine (3.0 mmol), the aldehyde (0.5 mmol), and Et3SiOTf (0.875 mmol). The mixture was stirred 48 h at room temperature and purified by chromatography (SiO2). Entries 9–14: Ph3P was used in place of EtOPPh2.
Yields were determined by 1H NMR using DMF as a standard.
See Supporting Information for structures of the minor products (2a′-2p′).
Propene (1 atm) was used in place of Ar.
Reaction time 18 h.
Reaction temperature 35 °C.
Fivefold larger reaction scale.
ratio of 2:3.
ratio of 3: (2q+2q′).
