Table 5.
Kinetic equations and parametric assignments for β-amylase.
| Maltose formation from soluble starch | 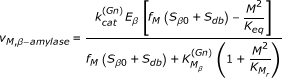 |
|---|---|
| Maltotriose formation from soluble starch | 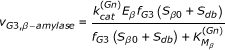 |
| Release of linear linkage groups from starch1 | 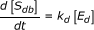 |
| Maltopentaose degradation to Maltose and Maltotriose | 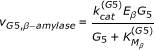 |
The kinetic model includes formation of maltose and maltotriose from starch and maltopentaose. β-Amylase turnover numbers 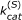 and
and 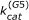 , mass concentration Eβ, equilibrium constant between maltose and maltotetraose Keq, Michaelis constants
, mass concentration Eβ, equilibrium constant between maltose and maltotetraose Keq, Michaelis constants 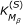 and
and 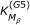 , inhibition constant associated with maltotetraose formation
, inhibition constant associated with maltotetraose formation 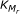 , mass concentration of debranching enzyme Ed, and rate constant for debranching kd are taken from reference [29]. M denotes the mass concentration of maltose, and terms containing M2 are related to β-amylase-catalyzed maltose condensation to yield maltotetraose.
, mass concentration of debranching enzyme Ed, and rate constant for debranching kd are taken from reference [29]. M denotes the mass concentration of maltose, and terms containing M2 are related to β-amylase-catalyzed maltose condensation to yield maltotetraose.
1 kd is bi-valued, with  for Sdb/Sdf < 0.3, and
for Sdb/Sdf < 0.3, and 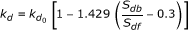 for Sdb/Sdf ≥ 0.3.
for Sdb/Sdf ≥ 0.3.
