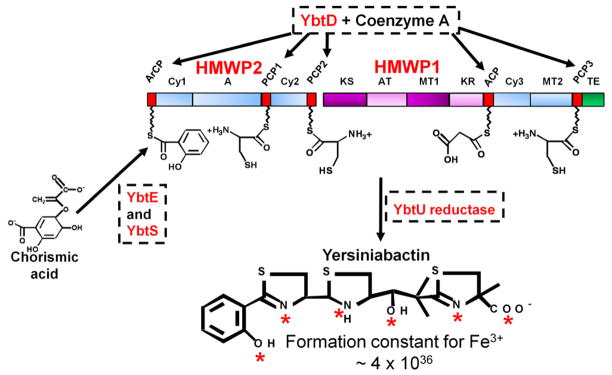
Fig. 1.
Biosynthesis of Yersiniabactin (Ybt). YbtD, a phosphopantetheinyl transferase, transfers the 4′-phosphopantetheine moiety of coenzyme A to carrier domains (red boxes) ArCP, PCP1, PCP2, ACP, and PCP3 which are attachment sites on HMWP1 and HMWP2 for the substrates adenylated salicylic acid, three cysteines, and malonate. Chorismic acid is converted to salicylic acid by YbtS. Salicylic acid is then adenylated by YbtE for attachment to the ArCP site of HMWP2. HMWP2 domains cyclize and condense two cysteines to form two thiazoline rings linked to the salicylate moiety while HMWP1 domains add the malonyl linker and convert a third cysteine to the final thiazoline ring. YbtU reduces the middle thiazolidine ring to a thiazoline ring to yield the final structure. Nonribosomal peptide synthetase (NRPS) domains are represented by blue boxes while polyketide synthase (PKS) domains are in purple. Aberrant or mischarged molecules on the enzyme complex are removed by YbtT, an editing thioesterase (not shown in diagram). The completed siderophore is released from the enzyme complex by the thioesterase (TE; green box) domain of HMWP1. The completed Ybt structure is shown with red asterisks identifying coordination sites for one Fe3+ atom. The disassociation constant (KD) for Fe3+ is shown. HMWP1 and HMWP2 enzymatic domains: A, adenylation; ACP, acyl carrier protein; ArCP, aryl carrier protein; AT, acyltransferase; Cy, condensation/cyclization; KR, β-ketoreductase; KS, ketoacyl synthase; PCP, peptidyl carrier protein; MT, methyltransferase; TE, thioesterase. The diagram is reproduced with modifications from Miller et al [31] with the permission of the Society for General Microbiology.