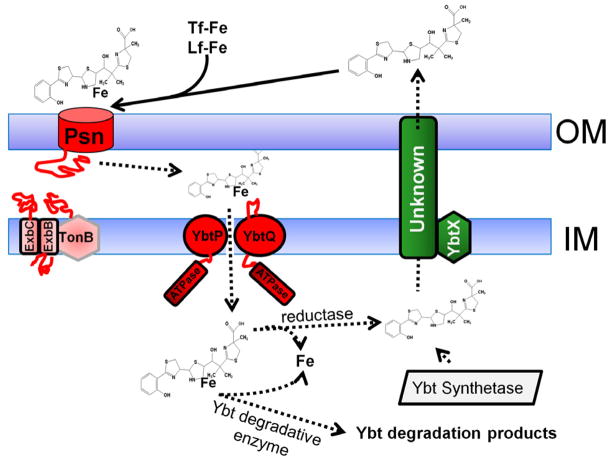
Fig. 2.
Ybt secretion and uptake. After release of the completed siderophore from the biosynthetic enzyme complex, Ybt is secreted from the cell by an unknown mechanism (green boxes). While there is evidence suggesting that YbtX is involved in secretion, it is not essential to this process. After release, Ybt can remove iron from transferrin (Tf-Fe) and lactoferrin (Lf-Fe). Although only uptake of radiolabeled iron has been experimentally demonstrated, our model favors uptake of the Fe-Ybt complex into the cell. The first step is binding to the TonB-dependent receptor Psn followed by translocation through the OM. Once in the periplasm Fe-Ybt is transported into the cytoplasm by YbtP/YbtQ. Whether additional components for this ABC transporter (e.g., a PBP or membrane spanning protein) are required for this step is undetermined. In the cytoplasm, Fe could be released from the siderophore by a reduction of Fe3+ to Fe2+ or by degradation of Ybt. Uptake components are in red with red lines denoting periplasmic and cytoplasmic domains. Dashed arrows indicate substrates or steps that are undetermined experimentally. The diagram is reproduced with modifications from Miller et al [31] with the permission of the Society for General Microbiology.