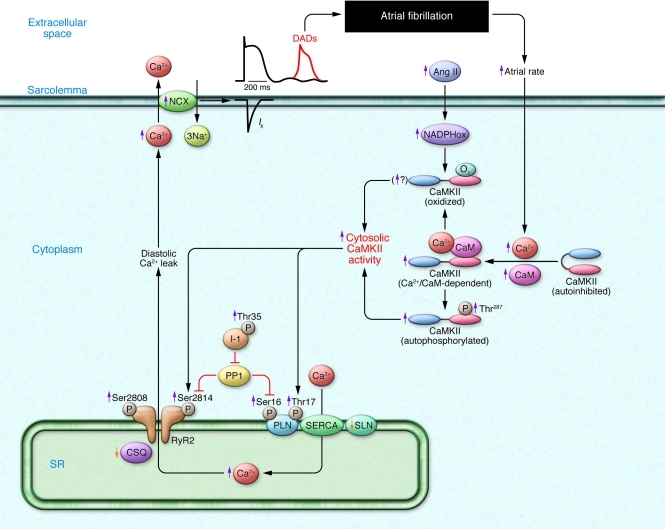
Figure 6. Factors promoting AF by inducing SR diastolic Ca2+ leak.
RyR dysfunction may result from hyperphosphorylation or exposure to excess intraluminal Ca2+. SR Ca2+ overload can result from phospholamban hyperphosphorylation or reduced sarcolipin (SLN) expression, which disinhibit SERCA and enhance SR Ca2+ uptake. Increased cellular Ca2+ entry due to high atrial rate facilitates Ca2+/calmodulin (CaM) binding to the regulatory domain of CaMKII, resulting in disinhibition of the catalytic subunit. After initial activation of the holoenzyme by Ca2+/CaM, oxidation at Met281/282 or phosphorylation at Thr287 blocks reassociation of the catalytic domain, yielding persistent CaMKII activity. PP1 suppression by enhanced SR compartment inhibitor–1 (I-1 activity) contributes to increased phospholamban (PLN) and RyR phosphorylation. NADPHox, oxidized NADPH.