Telomeres are repeated DNA sequences localized at the end of chromosomes and involved in maintaining chromosomal stability and integrity.1 Telomere length (TL) is considered a reliable marker of cell turnover, as telomeres progressively shorten with aging and cell proliferation. In various hematologic malignancies, hematopoietic cells have been shown to be characterized by shortened telomeres.1 So far, a few studies have evaluated the role of TL in myeloproliferative neoplasms (MPN).2–6
We analyzed TL in a cohort of MPN patients in order to investigate its relationship with biological and clinical parameters. We studied 139 consecutive MPN patients, including 42 cases of polycythemia vera (PV), 63 essential thrombocythemia (ET), 18 primary myelofibrosis (PMF), 12 post-PV MF, 4 post-ET MF followed at the Department of Hematology Oncology, Fondazione IRCCS Policlinico San Matteo, University of Pavia, Italy. Results were compared with findings in a cohort of 65 healthy subjects. This study was approved by the Ethics Committee, Fondazione IRCCS Policlinico San Matteo, Pavia, and the procedures followed were in accordance with the Helsinki Declaration of 1975, as revised in 2000.
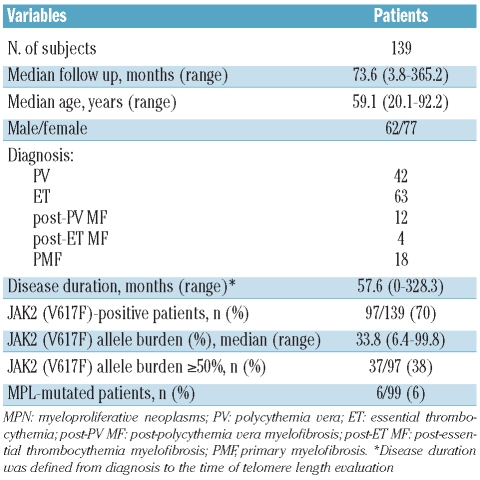
Demographic and hematologic characteristics of patients are listed in Table 1. TL was measured in both peripheral blood (PB) granulocytes and lymphocytes by flow-FISH, as previously described,7 with at least 30,000 events acquired for each sample. The age-corrected value of TL in granulocytes (deltaTELgran) was obtained by subtracting the individual patient’s measurement from the linear regression line established for healthy subjects: consequently, the shorter the patient’s TL value, the more negative the deltaTELgran value.
Table 1.
Demographic and hematologic characteristics of 139 MPN patients at the time of telomere length evaluation.
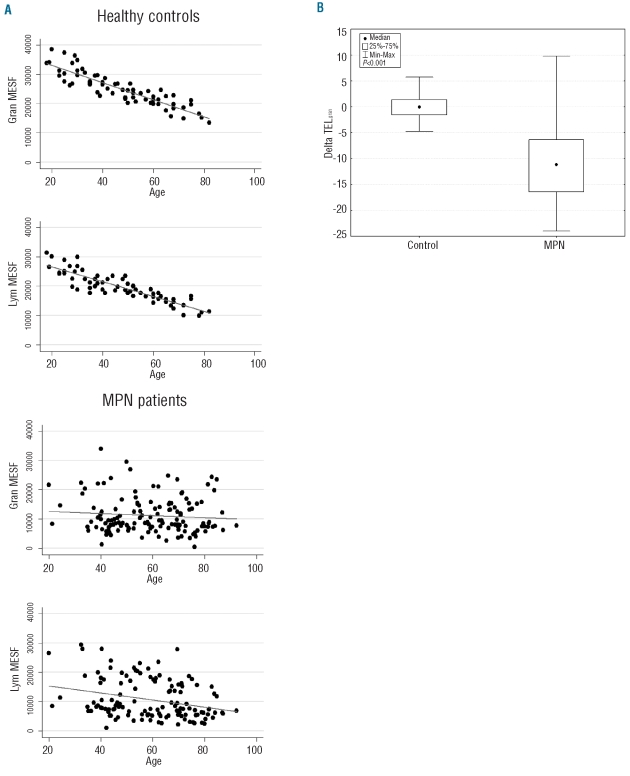
As a first step, we evaluated telomere fluorescence in PB granulocytes and lymphocytes, expressed as Molecular Equivalent of Soluble Fluorescence (MESF), from MPN patients and compared it to that of control subjects. In healthy individuals a significant negative linear correlation was found between telomere fluorescence of both granulocytes and lymphocytes and age (Spearman’s rho −0.90; P<0.001), whereas in MPN patients this relationship was retained in lymphocytes (r −0.30; P<0.001) but lost in granulocytes (r −0.07; P=0.40) (Figure 1A), suggesting that in MPN patients the impact of a clonal hematopoiesis exceeds the expected effect of demographic parameters on telomere shortening.
Figure 1.
Dynamics of telomere shortening in patients with myeloproliferative neoplasm (MPN) and in healthy subjects. (A) In healthy controls Spearman’s rank test revealed a significant negative linear correlation between telomere fluorescence, expressed as Molecular Equivalent of Soluble Fluorescence (MESF), and age both in peripheral blood (PB) granulocytes and lymphocytes (P<0.001). In contrast, in MPN patients this relationship was present in PB lymphocytes (P<0.001) but not in granulocytes (P=0.40). (B) Age-corrected telomere length (DeltaTELgran) is significantly shorter in MPN patients (median value −11.14, range −23.99 to 9.78) than in healthy individuals (−0.05, −4.74 to 5.75; Kolmogorov-Smirnov’s test P<0.001).
When considering the age-corrected value, patients with MPN showed a significantly lower deltaTELgran (median value −11.14, range −23.99 to 9.78) than healthy individuals (−0.05, − 4.74 to 5.75; P<0.001) (Figure 1B). In keeping with published data,4,5 we did not find any significant difference in deltaTELgran among MPN subtypes (P=0.249): median deltaTELgran in PV patients was –11.09 (25th percentile −16.90, 75th percentile −7.30), in ET −11.43 (−17.04 to −7.01) and in PMF, post-PV MF and post-ET MF −11.44 (−16.16 to −1.18). Moreover, no significant difference was observed between deltaTELgran in chronic phase of PV and ET and in their relative fibrotic evolution (P=0.64 and P=0.84, respectively). The wide variability in TL observed in PMF patients might reflect the heterogeneity of biological and pathogenetic features of this disease, even if further data from a larger cohort of patients are needed to investigate this observation thoroughly. In contrast, disease duration significantly affected deltaTELgran (Spearman’s rho −0.16; P=0.049), also when adjusted by MPN subtypes in multivariate analysis (P=0.01).
Overall, 97 of 139 (70%) patients carried the JAK2 (V617F) mutation, evaluated with a quantitative AS-PCR.8,9 The deltaTELgran was similarly shortened in JAK2 (V617F)-positive and JAK2 (V617F)-negative patients. This result was confirmed both by analyzing MPN patients as a whole (P 0.81) and according to MPN subtype (ET P=0.69, PMF P=0.44). In contrast, no significant relationship was observed between deltaTELgran and percentage of mutant alleles, either when considering this latter as a continuous variable (Spearman’s rho 0.03; P=0.73), or when comparing patients with less than 50% or 50% or more mutant alleles (P=0.56). Furthermore, we did not found any difference in deltaTELgran between patients carrying an MPL mutation10 (6 out of 99 evaluable patients, 6%) and patients with wild-type MPL (P=0.46). Taken together, these data confirm that patients with MPN present shortened telomeres irrespective of their JAK2 or MPL mutational status, implying that different molecular mechanisms may affect stem cells in the same way, and suggest that telomere assessment may be of potential diagnostic value in the work up of suspected MPN.
Finally, we investigated the effect of cytoreductive therapy on telomere shortening. At the time of TL evaluation, 87 of 139 patients were on cytoreductive therapy, mainly hydroxyurea. Our analysis showed that deltaTELgran was not affected by cytoreductive therapy per se (P=0.93) nor by its duration (Spearman’s ρ −0.07; P=0.42). Future evaluation of the impact of interferon α11 or innovative drugs12 on TL in MPN might be of interest.
Acknowledgments
This study was supported by Associazione Italiana per la Ricerca sul Cancro (AIRC, Milan) Special Program Molecular Clinical Oncology "5 per mille", and by Fondazione Cariplo, Milan, Italy.
Footnotes
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Calado RT, Young NS. Telomere diseases. N Engl J Med. 2009;361(24):2353–65. doi: 10.1056/NEJMra0903373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferraris AM, Pujic N, Mangerini R, Rapezzi D, Gallamini A, Racchi O, et al. Clonal granulocytes in polycythaemia vera and essential thrombocythaemia have shortened telomeres. Br J Haematol. 2005;130(3):391–3. doi: 10.1111/j.1365-2141.2005.05618.x. [DOI] [PubMed] [Google Scholar]
- 3.Terasaki Y, Okumura H, Ohtake S, Nakao S. Accelerated telomere length shortening in granulocytes: a diagnostic marker for myeloproliferative diseases. Exp Hematol. 2002;30(12):1399–404. doi: 10.1016/s0301-472x(02)00969-4. [DOI] [PubMed] [Google Scholar]
- 4.Spanoudakis E, Bazdiara I, Pantelidou D, Kotsianidis I, Papadopoulos V, Margaritis D, et al. Dynamics of telomere's length and telomerase activity in Philadelphia chromosome negative myeloproliferative neoplasms. Leuk Res. 2011;35(4):459–64. doi: 10.1016/j.leukres.2010.07.042. [DOI] [PubMed] [Google Scholar]
- 5.Bernard L, Belisle C, Mollica L, Provost S, Roy DC, Gilliland DG, et al. Telomere length is severely and similarly reduced in JAK2V617F-positive and -negative myeloproliferative neoplasms. Leukemia. 2009;23(2):287–91. doi: 10.1038/leu.2008.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruella M, Salmoiraghi S, Risso A, Carobbio A, Sivera P, Ricca I, et al. Telomere length in Ph -negative chronic myeloproliferative neoplasms: it is reduced according to JAK2 V617F mutation allele burden and it is not affected by cytoreductive treatment with hydroxyurea. ASH Annual Meeting Abstracts. 2010;116:1975. [Google Scholar]
- 7.Rufer N, Brummendorf TH, Kolvraa S, Bischoff C, Christensen K, Wadsworth L, et al. Telomere fluorescence measurements in granulocytes and T lymphocyte subsets point to a high turnover of hematopoietic stem cells and memory T cells in early childhood. J Exp Med. 1999;190(2):157–67. doi: 10.1084/jem.190.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Passamonti F, Rumi E, Pietra D, Della Porta MG, Boveri E, Pascutto C, et al. Relation between JAK2 (V617F) mutation status, granulocyte activation, and constitutive mobilization of CD34+ cells into peripheral blood in myeloproliferative disorders. Blood. 2006;107(9):3676–82. doi: 10.1182/blood-2005-09-3826. [DOI] [PubMed] [Google Scholar]
- 9.Passamonti F, Rumi E, Pietra D, Elena C, Boveri E, Arcaini L, et al. A prospective study of 338 patients with polycythemia vera: the impact of JAK2 (V617F) allele burden and leukocytosis on fibrotic or leukemic disease transformation and vascular complications. Leukemia. 2010;24(9):1574–9. doi: 10.1038/leu.2010.148. [DOI] [PubMed] [Google Scholar]
- 10.Pietra D, Brisci A, Rumi E, Boggi S, Elena C, Pietrelli A, et al. Deep sequencing reveals double mutations in cis of MPL exon 10 in myeloproliferative neoplasms. Haematologica. 2011;96(4):607–11. doi: 10.3324/haematol.2010.034793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kiladjian JJ, Cassinat B, Turlure P, Cambier N, Roussel M, Bellucci S, et al. High molecular response rate of polycythemia vera patients treated with pegylated interferon α-2a. Blood. 2006;108(6):2037–40. doi: 10.1182/blood-2006-03-009860. [DOI] [PubMed] [Google Scholar]
- 12.Pardanani A, Vannucchi AM, Passamonti F, Cervantes F, Barbui T, Tefferi A. JAK inhibitor therapy for myelofibrosis: critical assessment of value and limitations. Leukemia. 2011;25(2):218–25. doi: 10.1038/leu.2010.269. [DOI] [PubMed] [Google Scholar]