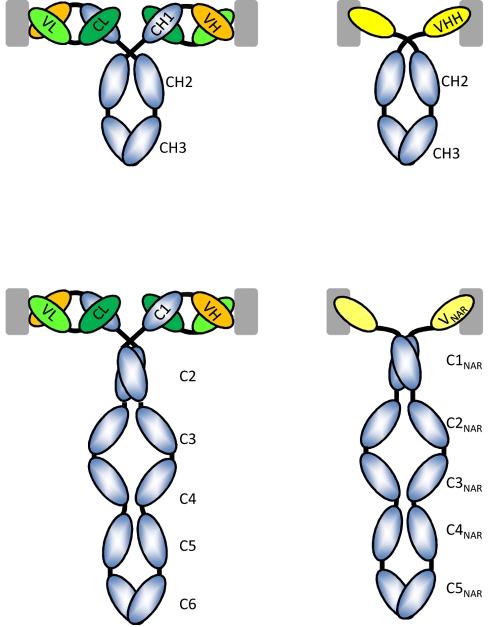
Figure 1. Schematic of classical H2L2 and H2 homodimeric HCAbs.
The left column displays the classical antibodies with two identical H-chains and two identical L-chains as they occur in mammals (IgG, top) and cartilaginous fish (IgW, bottom). The L-chain is in green and the antigen-binding site is formed by the paired VH and VL domains. The top right figure is the HCAb as it occurs in sera of camelids, the CH1 domain is missing and there is no L-chain. The antigen-binding site consists of one single domain, known as VHH. The H-chain of the IgW comprises six C domains and a variable domain at the N-terminal end, whereas the IgNAR (bottom right) is a homodimer of a H-chain with five C domains and a V-NAR at its N-terminal end. Note that the equivalent of the first C domain is absent. All of the antibodies are bivalent and the recognition of a possible antigen (gray square) is shown. The VH-VL associated preferentially with flat surfaces on the antigen, whereas the VHH or V-NAR has a preference to interact with cavities on the surface of the antigen.