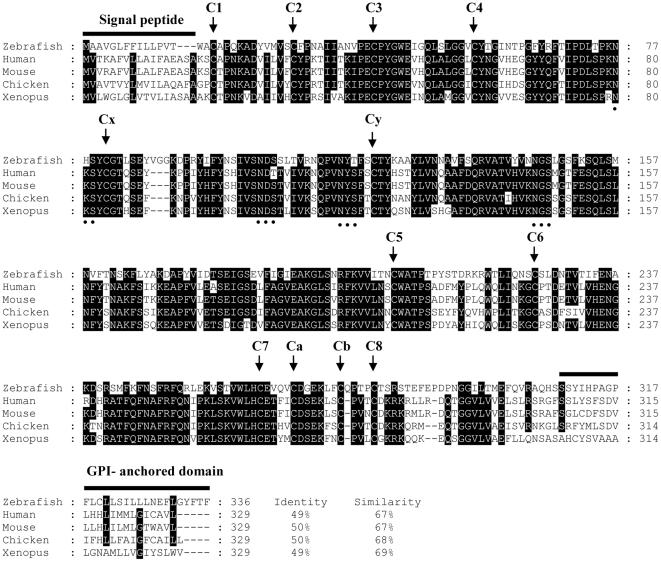
Figure 1. Zebrafish β-tectorin amino acid sequence alignment with those of other species.
Deduced amino acid sequences of zebrafish β-tectorin were aligned with those of human, mouse, chicken, and Xenopus. All β-tectorin proteins contained a conserved zona pellucida (ZP) domain of approximately 260 amino acids, with 12 highly conserved cysteine residues (indicated by arrows). Identical residues in 4 or 5 proteins are highlighted. Signal peptide and putative GPI-anchored domains are heavily overlined. The putative N-linked glycosylation sites are indicated by dots (…). The accession numbers of each β-tectorin from different species are listed below: human (XM_521604), mouse (X99806), chicken (AAA92461), and Xenopus (CAJ82963).