Figure 1.
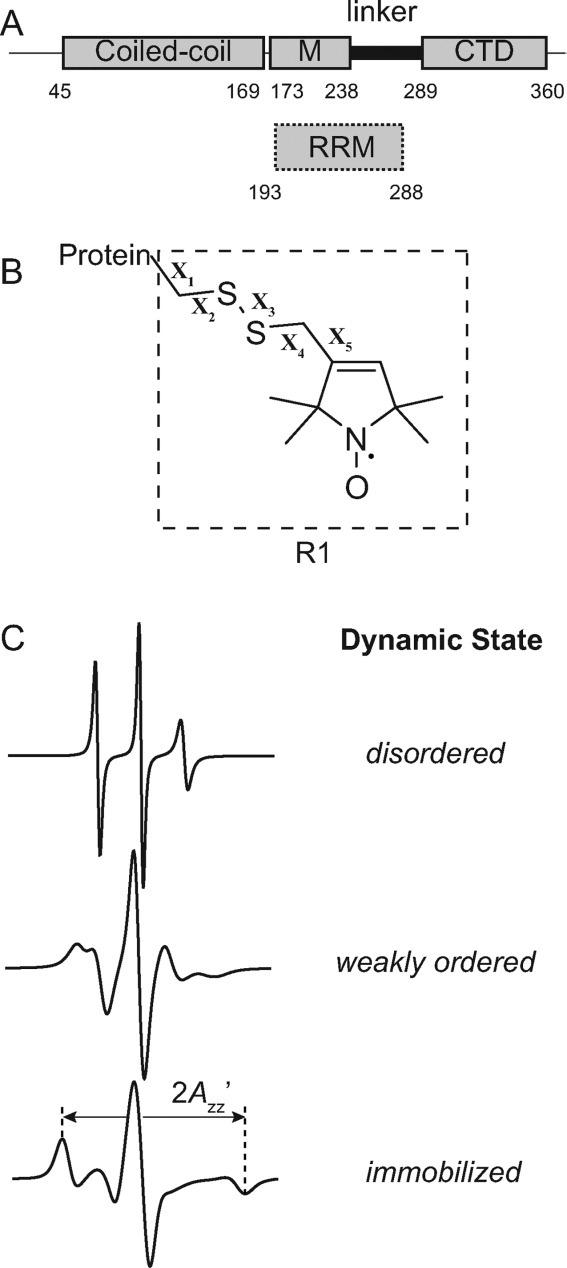
Schematic of ORF1p, the R1 spin label, and representative EPR spectra. (A) The top image displays a schematic of the ORF1p protein from the mouse L1 retrotransposon. Regions of the protein previously shown to be partially resistant to trypsin digestion are indicated by rectangles and correspond to a coiled-coil region, a middle region (M) and a C-terminal domain (CTD). The segment connecting the CTD and middle region is susceptible to trypsin proteolysis and is referred to as the linker. The lower image indicates where the presumed RNA recognition motif (RRM) binding domain is located based on the recently determined crystal structure of this domain from the human ORF1p protein (hORF1p). The amino acid number defining the beginning and end of each element is indicated. (B) Structure of the R1 side chain showing the dihedral angle designations (X1–X5). (C) Simulated EPR spectra corresponding to three fundamental dynamic modes of the R1 side chain in proteins: disordered (top trace), weakly ordered (middle trace), and immobilized (lower trace). Simulations were carried out using the NLSL.MOMD program21 available at (http://www.acert.cornell.edu/index_files/acert_ftp_links.php). In each case, the order parameter (S) and correlation time (τ) for the motion are given in the format {S,τ}. Details of spectral simulations are presented by Columbus et al.22
