Figure 3.
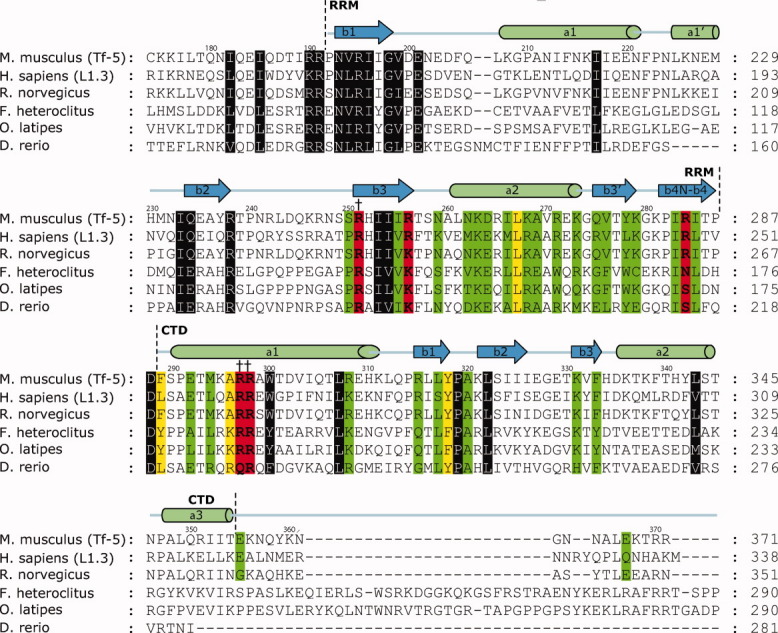
Primary sequence alignment of ORF1 proteins and a summary of the RNA binding and in vivo results. The alignment of amino acids of ORF1p contains the RRM identified by crystallography and the CTD identified by NMR. Amino acids that were mutated to cysteine or alanine residues and determined for their ability to bind a 60-mer L1 RNA molecule are color coded: green (KD < 3X wild type), yellow (3X wild type ≤ KD ≤ 6.1X wild type), and red (KD > 6.1X wild type). Residues that are highly conserved but were not mutated are colored gray. Mutants that disrupted in vivo retrotransposition are indicated by daggers. The ORF1 protein alignments were generated for mouse (from the Tf-5 retrotransposon, AAC53541.1), human (from the human L1.3 retrotransposon, AAB59367), rat (S21345), mumichog (AF055640), Zebrafish (CAD61093), and medaka (AAS83199) using CLUSTALW.36
