Figure 6.
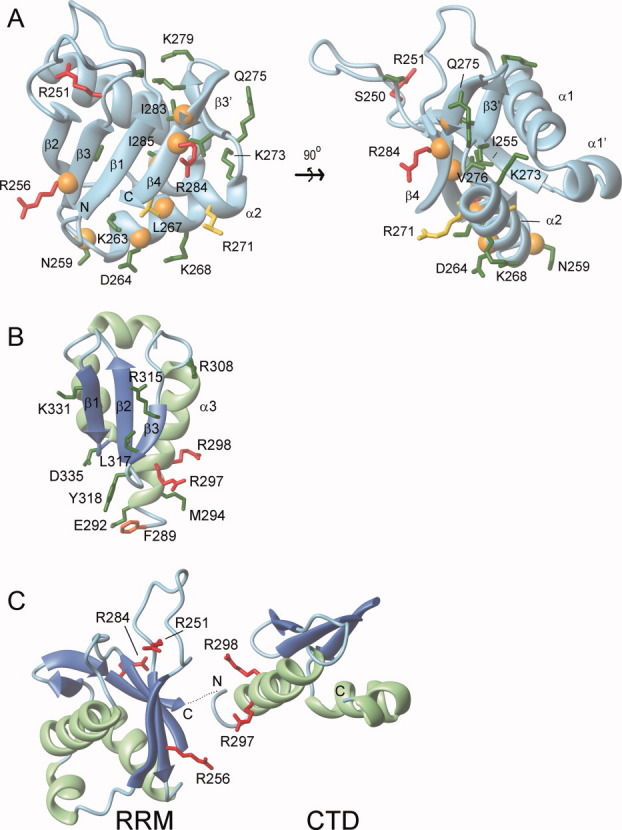
RNA binding model for the RRM and CTD. Residues important for RNA binding within the (A) RRM domain and (B) CTD. Amino acids within ORF1p that were mutated to cysteine or alanine residues and determined for their ability to bind a 60-mer L1 RNA molecule are color coded: green (KD < 3X wild type), yellow (3X wild type ≤ KD ≤ 6X wild type), and red (KD > 6X wild type). An orange sphere positioned at the alpha carbon atom indicates R1 labeled cysteine mutants that exhibited RNA-dependent changes in their EPR spectra. (C) Proposed primary interaction surface with RNA. The CTD and RRM domain are positioned adjacent to one to form a continuous surface that interacts with single stranded RNA. It is unclear whether this surface is formed by the RRM and CTD from a single monomer or from different monomers within the trimer. The orientation of the RNA molecule on this surface is also unknown. In panels B and C, ribbon diagrams of the RRM domain and CTD are shown with the sheet and helices colored blue and green, respectively. The secondary structure topology is labeled accordingly for the RRM (PDB 2w7a)23 and the CTD (PDB 2jrb).20
