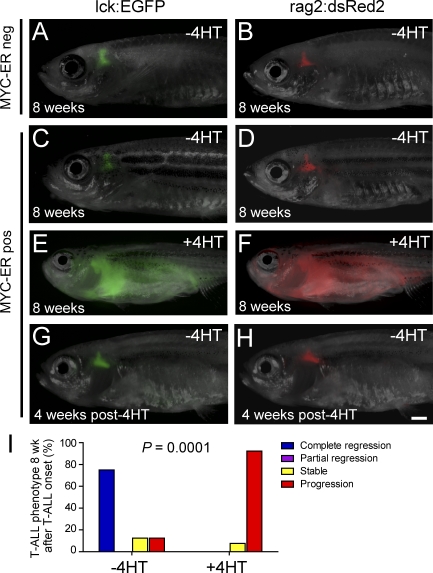
Figure 1.
Conditional T-ALL development in rag2:MYC-ER transgenic zebrafish. (A and B) Thymic fluorescence in control MYC-ER–negative lck-EGFP transgenic (A) and rag2:dsRed2 transgenic (B) zebrafish raised in the absence of 4HT. (C and D) Thymic fluorescence in the absence of 4HT treatment in rag2:MYC-ER transgenic zebrafish that also expressed lck-EGFP (C) or rag2:dsRed2 (D). For A–D, one representative zebrafish is shown from a minimum of eight fish raised in each condition. (E and F) Fully penetrant T-ALL in rag2:MYC-ER transgenic zebrafish raised in 50 µg/liter (129 nM) 4HT. A representative triple-transgenic rag2:MYC-ER; lck:EGFP; rag2:dsRed2 zebrafish is shown at the time of disseminated T-ALL development, imaged in both green and red fluorescent channels. (G and H) Thymic fluorescence in the rag2:MYC-ER transgenic zebrafish from E and F, shown 4 wk after 4HT removal. In all triple-transgenic zebrafish in which regression occurred (n = 6 of 8), T-ALL regression occurred simultaneously in both green and red fluorescent channels, with no evidence of residual EGFP-positive dsRed2-negative mature T cells, indicating that differentiation was not the primary mechanism of T-ALL regression. Bar, 1 mm. (I) After T-ALL development, zebrafish were either removed from 4HT to down-regulate the MYC transgene (−4HT) or kept in 4HT (+4HT), and tumor phenotype was assessed 8 wk after 4HT removal. Zebrafish that became moribund with leukemia before the 8-wk time point were euthanized and classified into the progression category. Number of fish analyzed per condition: −4HT, n = 8; +4HT, n = 13.