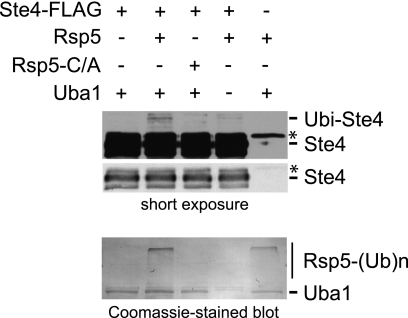
FIGURE 4.
Rsp5 ubiquitinates Ste4 in vitro. Bead-immobilized Ste4-FLAG was incubated with Rsp5 or catalytically inactive Rsp5 (Rsp5-C/A) in the absence or presence of E1 activating enzyme (Uba1). All reactions contained Ubc5b, ATP, and ubiquitin. Reactions were stopped after 30 min with 2% SDS. Proteins were separated by 10% SDS-PAGE, transferred to nitrocellulose membranes, and blotted with M2 anti-FLAG antibody and goat-anti-mouse-HRP secondary antibody. Monoubiquitination slows the electrophoretic mobility of Ste4-FLAG by the approximate mass of ubiquitin (∼8 kDa). Long (top panel) and short exposures (center panel) are shown. After immunoblotting, the nitrocellulose blot was stained with Coomassie to show Rsp5 autoubiquitination and Uba1 loading (bottom panel). *, antibody heavy chain. Because we observed that FLAG peptide elution buffer inhibited Rsp5 catalytic activity, we conducted in vitro ubiquitination with bead-immobilized Ste4-FLAG, which may also reduce reaction efficiency.