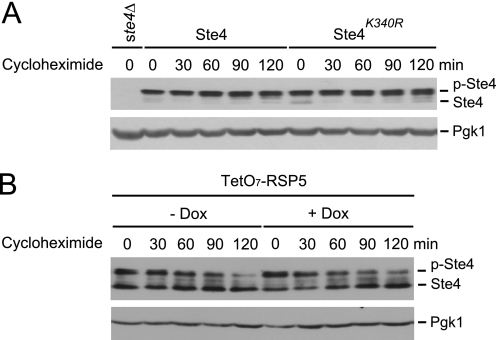
FIGURE 6.
Blocking ubiquitination does not alter the stability of Ste4. A, cells expressing either wild-type Ste4 or Ste4K340R were grown to early log phase, treated with 3 μm pheromone for 1 h, and followed by treatment with the protein synthesis inhibitor cycloheximide for the indicated times. The level of Ste4 and Ste4K340R was detected by immunoblotting (IB). B, early log TetO7-RSP5 (RSP5 under the control of doxycycline-regulatable promoter) cells were first not-treated (-Dox) or treated with doxycycline (+Dox) for 9 h (to switch off RSP5 expression in the TetO7-RSP5 cells). Cells were then treated with 3 μm pheromone for 1 h, followed by treatment with the protein synthesis inhibitor cycloheximide for the indicated times. The abundance of Ste4 was detected by immunoblotting (IB). Equal loading of each lane was confirmed by immunoblotting with anti-Pgk1. Note that Ste4 is dephosphorylated more prominently in the TetO7-RSP5 strain, which resulted in the appearance of two bands.