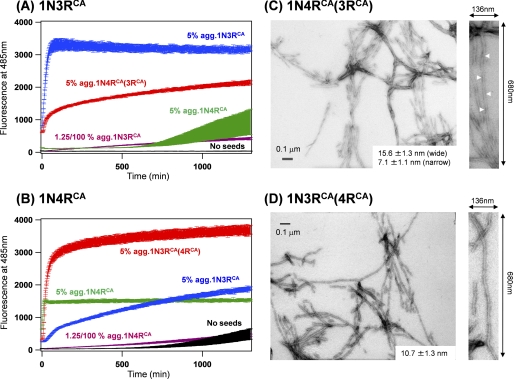
FIGURE 5.
Tau fibrils with alternative structural properties are emerged by a seeding reaction. A, a seeding reaction of 10 μm 1N3RCA in a 50 mm Tris buffer, pH 7.2, containing 1 mm EDTA, 2.5 μm heparin, and 16.7 μm ThT is monitored by a fluorescence increase of ThT in the absence of sample agitation. A kinetic trace in the absence of added seeds was also shown (black). As also shown in Fig. 4A, the addition of 0.5 μm 1N3RCA aggregates to soluble 1N3RCA significantly accelerates the fibrillation (blue trace). Although 1N4RCA aggregates (0.5 μm, green trace) did not efficiently function as seeds, 0.5 μm 1N4RCA(3RCA) aggregates significantly accelerated the fibrillation of 1N3RCA (red trace). This is not due to the residual 1N3RCA aggregates in the 1N4RCA(3RCA) aggregates, which was evidenced by an inefficient seeding of 1N3RCA fibrillation by adding 1.25 × 10−3 μm (1.25/100% amounts of soluble 1N3RCA) 1N3RCA aggregates (purple trace). B, a seeding reaction of 10 μm 1N4RCA in the conditions that are the same with those in A. Seeds added are as follows: 1N3RCA aggregates (0.5 μm, blue trace), 1N4RCA (0.5 μm, green trace; 1.25 × 10−3 μm, purple trace) and 1N3RCA(4RCA) aggregates (0.5 μm, red trace). A spontaneous fibrillation of 1N4RCA in the absence of agitation is also shown as a black trace. C and D, electron micrographs of cross-seeded Tau fibrils, 1N4RCA(3RCA) (C) and 1N3RCA(4RCA) (D). An average value of fibril widths is indicated, and a magnified image (136 × 680 nm) of a representative Tau fibril is also shown on the right side of each panel. White arrowheads indicate cross-over points of Tau fibrils with helical morphologies.