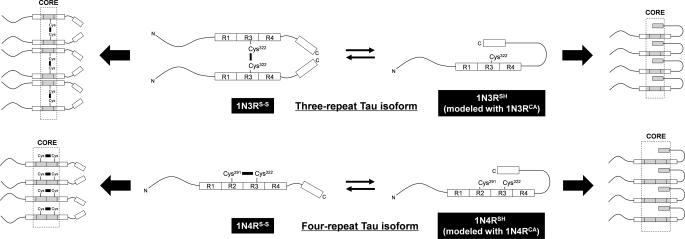
FIGURE 6.
A proposed mechanism to generate structural polymorphism of Tau fibrils. Three- and four-repeat Tau isoforms are schematically represented together with pseudo-repeats (R1, R2, R3, and R4) and a C-terminal region highlighted as boxes. N and C indicate the N and C termini, respectively. In both 1N3R and 1N4R isoforms, a disulfide-reduced state is assumed to allow the C-terminal region to interact with the repeat region (1N3RSH and 1N4RSH), although such interactions may be precluded by formation of either intermolecular (1N3R) or intramolecular (1N4R) disulfide (1N3RS-S and 1N4RS-S). An exact alignment of Tau molecules in a fibril remains unknown; therefore, alignment/orientation of boxes and Cys residues in the schematic representation is still speculative. Despite this, our results support that a C-terminal region is involved in the core of disulfide-reduced Tau (right side) but not in that of disulfide-bonded Tau (left side). Protease-resistant core regions (Fig. 3) are colored gray and also highlighted by dotted lines.