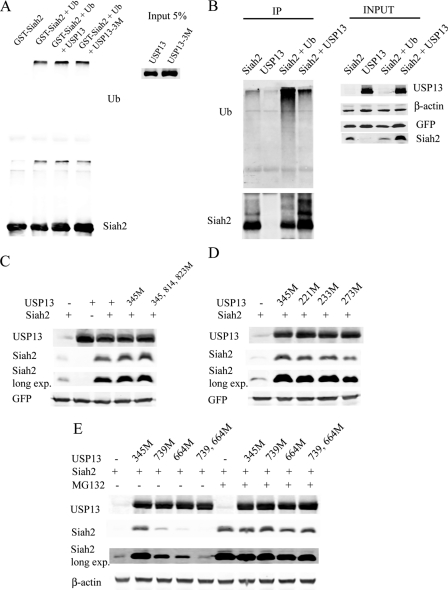
FIGURE 4.
USP13 regulates Siah2 protein stability via noncatalytic UBA domains. A, in vitro ubiquitination reactions for GST-Siah2 followed by deubiquitination assay in presence of USP13. The ubiquitination was monitored by Western blot analysis using anti-ubiquitin antibody. The amount of GST-Siah2 used in these reactions is shown in the lower panel using anti-Siah2 antibody. Ub, ubiquitin. B, 293T cells were transfected with FLAG-Siah2/Myc-USP13 and, where indicated, with a ubiquitin expression construct and lysed by a heat-denaturing method. Protein extracts were immunoprecipitated (IP) with anti-FLAG antibody and immunoblotted with anti-ubiquitin antibody. The amount of immunoprecipitated Siah2 was determined using anti-FLAG antibody. C and D, cells were co-transfected with a GFP-expressing vector, FLAG-Siah2, and USP13 wild-type or mutant constructs (M). After 24 h, the steady levels of FLAG-Siah2 and USP13 were detected with corresponding antibodies. Total cell lysates were analyzed for GFP expression to assess transfection efficiency. exp., exposure. E, cells were co-transfected with FLAG-Siah2 and USP13 wild-type or mutant constructs (M) for 24 h. Where indicated, the cells were incubated with MG132 (20 μm) for an additional 4 h. Cell lysates were prepared, and USP13 and FLAG-Siah2 levels were monitored by immunoblotting with anti-FLAG and anti-USP13 antibodies. β-Actin served as the loading control.