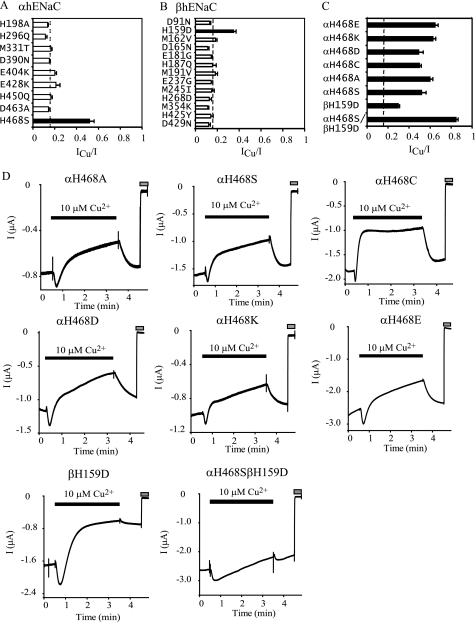
FIGURE 5.
Identification of αHis468 and βHis159 as potential sites mediating Cu2+ interaction with hENaCs. Point or double mutations were introduced in one hENaC subunit, which was co-expressed with other WT hENaC subunits. Mutational sites were selected to substitute nonconserved residues capable of binding Cu2+ in α or β hENaC subunits with their corresponding residues in α or β mENaC-based on sequence alignments. A, ICu/I at 10 μm Cu2+ from oocytes expressing mutant α and WT β and γ hENaCs (n = 5). The ICu/I values in this and all other subsequent figures were obtained in the same way as in Fig. 4F. B, ICu/I from oocytes expressing mutant β and WT α and γ hENaCs (n = 5 except n = 4 for D91N and D429N). C, ICu/I from oocytes expressing αHis468 mutants and αH468S/βH159D (n = 5–7). The black bars in A–C indicate that the values were significantly different from that of WT αβγ hENaCs obtained in the same batches of oocytes (p < 0.01). The dashed line shows the average ICu/I values from all oocytes expressing WT αβγ hENaCs used in this experiment (0.15 ± 0.00, n = 164) for reference only but not for statistical significance analyses. D, representative recordings showing the effect of 10 μm Cu2+ on mutant hENaCs. The gray bars indicate the times when 10 μm amiloride was present. Bath solution was buffered with 10 mm HEPES.