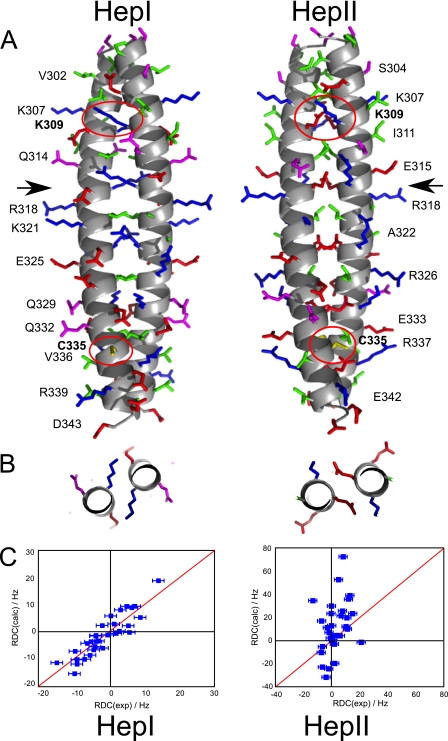
FIGURE 8.
A, model of the proposed conformations of the Nek2 leucine zipper. The leucine zipper for both conformations is shown with the N terminus at the top and the C terminus at the bottom. The main chain is shown as a helical scheme, the side chains are shown as stick models. No hydrogens are shown. Side chains are colored according to their properties as in Fig. 2. Residues selected for easy visibility are labeled to provide guidance. Arrows indicate the position of Arg-116 in HepI and Glu-317 in HepII where the slices shown in B are taken. Residues Lys-309 and Cys-335, which are mutated, are labeled in bold and the side chains encircled in red. B, packing of charged side chains in the A position of HepI (Arg-316) and the D position in HepII (Glu-317) in a slice through the center of the coiled-coil as marked by arrows in A. C, validation of the model by measurement of 1H-15N RDC values for mutant K309C/C335A of LZ5. Measured RDCs on the x-axis are compared with predicted RDCs (on the y-axis) calculated with PALES for the two models.