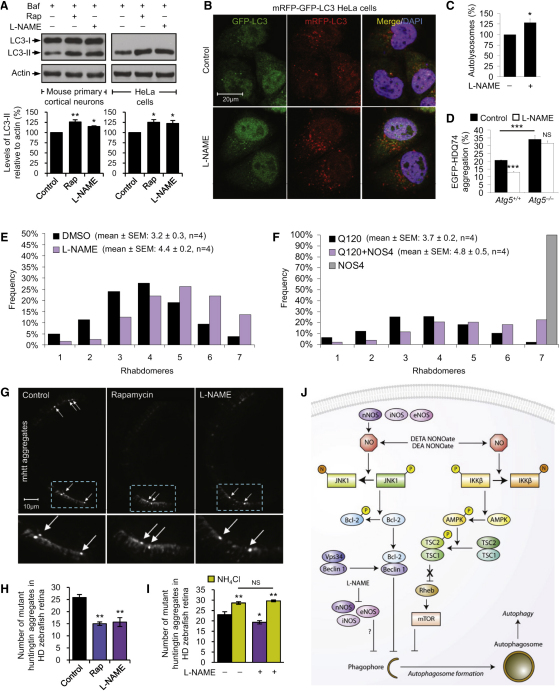
Figure 7.
L-NAME Induces Autophagy and Reduces Mutant Huntingtin Aggregation/Neurodegeneration in Models of Huntington's Disease
(A) Immunoblot analyses with anti-LC3 antibody show that rapamycin (8 hr) and L-NAME (24 hr) increased autophagosome synthesis in bafilomycin A1-treated mouse primary cortical neurons and HeLa cells.
(B and C) Confocal microscopy images (B) and analysis of autophagic flux by automated Cellomics microscope (C) show that L-NAME increased autolysosomes in mRFP-GFP-LC3 HeLa cells.
(D) L-NAME reduced EGFP-HDQ74 aggregates in EGFP-HDQ74–transfected Atg5+/+, but not in Atg5−/−, MEFs.
(E) Drosophila expressing mutant huntingtin exon 1 (Q120) shows a significant decrease in neurodegeneration (p < 0.001, paired t test) upon L-NAME treatment compared to DMSO.
(F) Expression of an endogenous negative regulator of NOS activity (NOS4) significantly attenuates neurodegeneration (p < 0.05, paired t test) in Drosophila expressing mutant huntingtin exon 1 (Q120).
(G–I) Images from the retina of transgenic HD zebrafish show mutant huntingtin aggregates (arrows) (G). Treatment with rapamycin or L-NAME reduced the number of aggregates (H). L-NAME did not reduce aggregates in the presence of NH4Cl, which increased aggregate count (I).
(J) NO inhibits autophagy by S-nitrosylating and inhibiting JNK1 phosphorylation, thereby reducing phospho-Bcl-2 and increasing Bcl-2–Beclin 1 interaction, which disrupts hVps34–Beclin 1 association. NO also S-nitrosylates and inhibits IKKβ phosphorylation, leading to reduced phospho-AMPK and TSC2 activity, which alleviates the inhibitory effect of TSC1/2 on Rheb (denoted by “×”), thereby allowing Rheb to activate mTORC1 and inhibit autophagy. Overexpression of NOS isoforms impairs autophagy by inhibiting the JNK1 pathway, whereas NOS inhibition by L-NAME induces autophagy by mechanism distinct from the NO pathways. Graphical data denote mean ± SEM, except in (E) and (F).