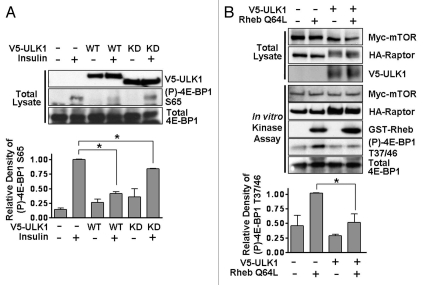
Figure 5.
ULK1 inhibits phosphorylation of 4E-BP1 by mTORC1. (A) HEK293 cells were transfected with wild-type or kinase-dead ULK1, serum-starved overnight and then stimulated with 100 nM insulin for 30 min prior to lysis, where indicated. Phosphorylation of 4E-BP1 at Ser65 was analyzed by western blotting. Total 4E-BP1 and ULK1 are shown as controls. Densitometry of phospho-4E-BP1 from independent experiments is shown in the graph (mean ± SD). Statistical significance was analyzed using a student's t-test, *p < 0.01. (B) An in vitro mTORC1 kinase assay was performed by combining immunoprecipitated Myc-mTOR/HA-Raptor complexes, which were expressed with or without V5-ULK1, with recombinant GST-4E-BP1 in the presence or absence of active GST-Rheb (Q64L), as indicated. mTORC1 kinase activity was determined by western blotting using phospho-4E-BP1 (Thr37/46) antibody. The densitometry of the phospho-4E-BP1 blots from the three experiments is shown in the graph (mean ± SD). Statistical significance was analyzed using a student's t-test, *p < 0.05.