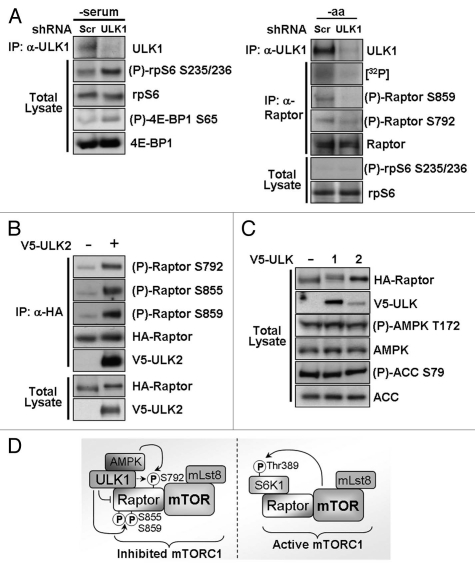
Figure 7.
Knockdown of endogenous ULK1 prevents Raptor phosphorylation and increases mTORC1 signaling. (A) Endogenous ULK1 expression was knocked down using shRNA and the phosphorylation of endogenous mTORC1 substrates was determined using phospho-specific antibodies (left part). Phosphorylation of Raptor was determined by [32P]-radiolabel incorporation into endogenous Raptor and using phospho-specific antibodies (right part). Scrambled (Scr) shRNA was used as a control. -serum = serum-free DMEM, -aa = amino acid- and serum-free Krebs Ringer Buffer. (B) V5-ULK2 and HA-Raptor were overexpressed in HEK293 cells. Following an HA-immunoprecipitation, phosphorylation of Raptor was determined using site-specific antibodies. Total Raptor levels are shown as a control. (C) Levels of endogenous phospho-AMPK (Thr172) and phospho-ACC (Ser79) were analyzed under normal growth conditions in HEK293 cells which had been transfected with V5-ULK1 or V5-ULK2 24 h prior to lysis. Total AMPK, ACC, HA-Raptor and V5-ULK1 are shown as controls. (D) A schematic diagram showing the proposed mechanism of ULK1-mediated inhibition of mTORC1. ULK1 inhibits signal transduction through mTORC1 (mTOR/Lst8/Raptor protein complex) by interaction with and phosphorylation of Raptor (ULK1 P-sites: Ser855, Ser859 and weakly at Ser792). ULK1 interaction with Raptor interferes with mTORC1 substrate recognition. AMPK, which binds to ULK1, also phosphorylates the inhibitory Ser792 site. Upon repression of autophagy, ULK1 dissociates from Raptor allowing Raptor to efficiently interact and phosphorylate mTORC1 substrates such as S6K1 on Thr389.