Figure 4.
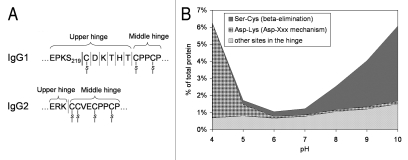
(A) Schematic drawing of a portion of IgG1 and IgG2 hinge region indicating sites of fragmentation. (B) pH-dependence of IgG1 hinge fragmentation monitored by SEC. Fragmentation between S219 and C220 in IgG1 that proceeds via β-elimination is shown as a solid line. The rest of the cleavages are shown as dotted lines. Disulfide bridges are indicated. The samples in (B) were incubated for approximately two weeks at 45°C. To identify the cleavage sites, the Fab fragments were purified by SEC and then analyzed by LC/MS. Considering the relatively wide pH range of 4–10, the cleavage sites can be divided into three groups. (1) Ser-Cys bond, where cleavage occurs via beta elimination, becomes dominant at higher pH. (2) Asp-Lys bond, where cleavage dramatically increases at pH < 5, presumably involving Asp side chain. (3) The rest of the cleavage sites in the hinge, where pH dependence is not as remarkable.