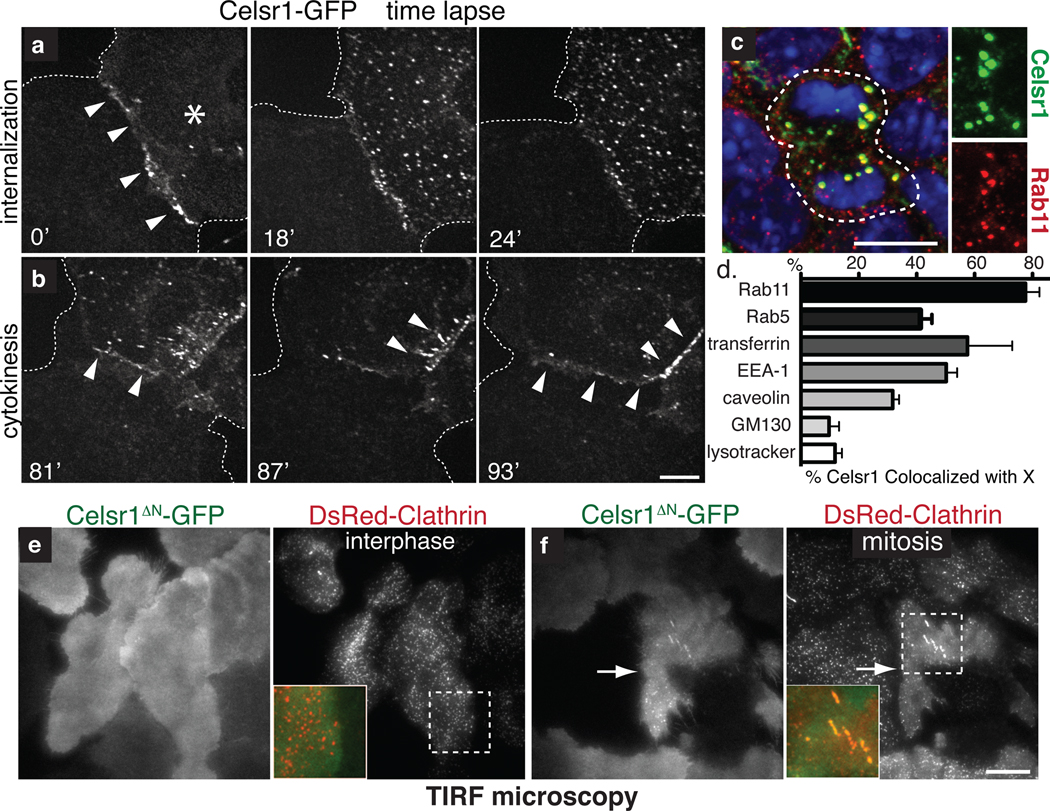
Fig. 3. Upon mitosis, PCP components are endocytosed by a clathrin-dependent mechanism and recycled to the plasma membrane.
(a) Time-lapse images of Celsr1-GFP internalization in a live mitotic keratinocyte in vitro. Cell on the right (asterisk) is undergoing mitosis. Note that Celsr1 localizes to the cell-cell contact during interphase, but internalizes as cell enter mitosis. (b) Time-lapse images of Celsr1-GFP redelivery to sites of intercellular contacts towards the end of mitosis (see Supplementary Movie 1). (c) Celsr1 co-localizes with Rab11, a marker of recycling endosomes, in mitotic basal cells in vivo. Planar confocal sections through the basal layer of E15.5 backskin labelled with Celsr1 (red) and Rab11 (green) antibodies. Right panels show individual channels. Chromatin is labelled with DAPI (blue). (d) Celsr1-GFP co-localizes appreciably with Rab11, early endosome markers Rab5 and EEA-1, and internalized transferrin, a marker of clathrin-dependent endocytosis. Some overlap is observed with caveolae marker caveolin. By contrast, Celsr1-GFP is largely independent of golgi marker GM130, and lysosomal marker lysotracker in mitotic keratinocytes in vitro. Shown is the mean percentage of Celsr1 co-localized with X per cell. n=4–10 cells for each marker. Error bars denote s.e.m. (see Fig. S2 for images). (e–f) Celsr1 internalization is increased during mitosis. TIRF microscopy was performed on live keratinocytes expressing Celsr1ΔN-GFP and DsRed-Clathrin. During interphase Celsr1ΔN-GFP is stably associated at the surface (e). In mitosis, Celsr1ΔN-GFP forms surface puncta which co-localize with DsRed-Clathrin (see quantifications in Fig. 4l and Supplementary Movies 3–4). Bars=10µm.