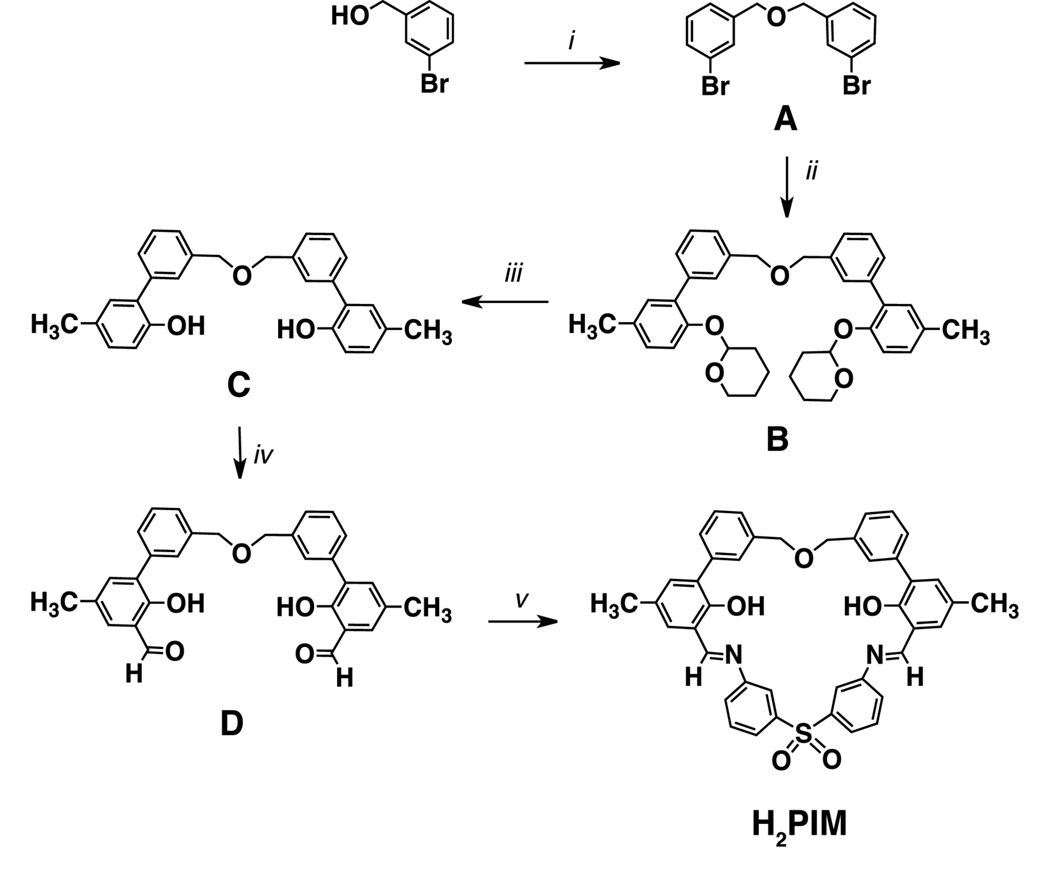
Scheme 2.
i. a) NaH, dry THF, b) 3-bromobenzylbromide; ii. a) Aryl zinc reagent: 2-(2-bromo-4-methylphenoxy)-tetrahydro-2H–pyran, n-butyllithium, ZnCl2, THF, b) Pd(PPh3)4; iii. oxalic acid, THF/MeOH (1:1), 50 °C; iv. anhydrous MgCl2, paraformaldehyde, NEt3, CH3CN, reflux; v. 2,2’-bis(aminophenyl)sulfone, trifluoroacetic acid, dry CH3CN.