Abstract
Purpose
Telomerase-immortalized human corneal epithelial cells have been reported to stratify and differentiate in vitro similar to native tissue. The purpose of this study was to assess the ability of a telomerase-immortalized human corneal epithelial cell line to generate a full thickness epithelium in vivo in athymic mice.
Methods
Telomerized corneal epithelial cells were transduced with a retroviral vector encoding the herpes simplex thymidine kinase gene. Efficacy of the thymidine kinase suicide gene was confirmed using a live/dead assay. The epithelium was mechanically removed from athymic nude mice and remaining cells were treated with mitomycin C to prevent re-epithelialization. Telomerized corneal epithelial cells were seeded onto the denuded cornea and allowed to adhere for 4 and 24 hours. Cellular attachment was assessed using a fluorescent cell tracker. Stratification and differentiation were assessed after 7 days using phalloidin and a mouse monoclonal antibody to K3
Results
Telomerized corneal epithelial cells were visualized across the denuded stromal surface at 4 and 24 hours, with multi-layering evident at the latter time point. No epithelium was present in the non-treated eye. 7 days post-transplantation cells stratified into a multilayered epithelium, with positive K3 expression in basal and suprabasal cells. Treatment with ganciclovir induced significant loss of viability in vitro.
Conclusions
The findings in this pilot study demonstrate that telomerized corneal epithelial cells possess the capacity to reconstitute a stratified corneal epithelium in vivo. The introduction of thymidine kinase allowed for the successful induction of cell death in proliferating cells in vitro. Collectively, these data suggest that a telomerase-immortalized corneal epithelial cell line transduced with thymidine kinase represents a potential model for studying differentiation and epithelial-niche interactions in vivo with potential applications in tissue engineering.
Keywords: cornea, epithelium, telomerase, differentiation, limbal niche
Introduction
The corneal epithelium is a five to seven cell layered stratified epithelial sheet that functions as a tight barrier to protect the eye from invading pathogens and provide a smooth refracting surface essential for vision. Maintenance of the epithelium is accomplished by a small population of lineage-restricted stem cells that reside within the basal layer in the limbus.1 Here slow cycling cells undergo continuous, error free division to generate progeny that will ultimately leave the niche and migrate centripetally across the corneal surface.2 These early progeny, or transient amplifying cells, retain significant proliferative capacity in the peripheral epithelium, exhibiting the highest number of mitotic divisions, and reduced capacity within the central cornea, where they undergo a final round of division and vertically migrate as paired daughter cells toward the ocular surface.3, 4 These terminally differentiated cells are ultimately shed from the surface through apoptotic signaling mechanisms into the precorneal tear film.5, 6
Currently available corneal epithelial cell lines include virally transformed cells using the SV-40 large T antigen, adenovirus E1A, and HPV16-E6/E7.7–10 The recent development of epithelial cell lines expressing telomerase have allowed for extension of cellular lifespan without disruption of normal differentiation programs.11 The advantages associated with corneal epithelial cells immortalized through forced expression of human telomerase reverse transcriptase (hTERT) are many, including genomic stability, retention of cell cycle regulatory pathways, and a normal repertoire of stratification and differentiation-associated gene products when cultured under sequential submersed/airlifted conditions in vitro.12 Specifically, human telomerized corneal epithelial (hTCEpi) cells have been shown to express the accepted cornea differentiation marker K3, the tight junction protein ZO-1, and the membrane associated mucin MUC1.12 Moreover, in airlifted culture, hTCEpi cells demonstrate loss of nuclear localized BCL2 prior to positive TUNEL labeling, suggestive of intact apoptotic signaling programs.12
The ability of an immortalized cell line to differentiate in vivo represents a useful model for studying mechanisms of corneal epithelial differentiation and regulatory factors responsible for the re-establishment of the niche microenvironment. Current air-lifted studies of corneal epithelial cell differentiation are limited by the absence of interactive stromal effects as well as critical factors in the precorneal tear film. The implementation of telomerase-immortalized cell lines for in vivo studies have the added therapeutic potential to allow for an unlimited cell supply in emerging tissue engineering techniques both within and outside the eye.13 While cell lines immortalized by telomerase have reportedly failed to form tumors in immunodeficient mice,14, 15 the potential for uncontrolled proliferation associated with the use of an immortalized cell line raises concerns which limit the usefulness of these models.16
Gene directed pro-drug enzyme therapy (GDEPT), also known as suicide gene therapy, is a technique currently being explored in the treatment of cancer.17–19 The advantage of GDEPT over traditional chemotherapy is that it allows for specific target-induced cell death, thereby reducing overall systemic effects. This target specificity is accomplished by combining a non-toxic pro-drug with a specific intracellular enzyme which is capable of converting the drug into the active form. Several drug/enzyme combinations for GDEPT have been examined.20 For the application of GDEPT in our tissue-engineering model, we investigated the efficacy of the ganciclovir/thymidine kinase (TK) system as a potential “kill switch” in our immortalized cell line. This classical system sensitizes the target cell to ganciclovir induced cell death after exogenous expression of the thymidine kinase gene from the herpes simplex virus by inhibiting DNA polymerase and induced ssDNA breaks during S-phase.17
In this pilot study, we investigated the ability of a telomerase-immortalized human corneal epithelial cell line transduced with a thymidine kinase suicide gene (HyTK) to adhere to a denuded corneal surface and undergo vertical stratification and differentiation in vivo; and assessed the ability of the HyTK gene to regulate viability following treatment with ganciclovir in vitro.
Materials and Methods
Cell culture
Human telomerase-immortalized corneal epithelial cells (hTCEpi) were cultured in 0.15 mM calcium KGM-2 serum-free culture media as previously described.12 Cells were subcultured on T75 tissue culture flasks (Falcon Labware; BD Biosciences, Bedford, MA), incubated at 37°C in 5% CO2 and passaged every 7 to 10 days. For establishment of the HyTK cell line, hTCEpi cells were transduced with a retroviral vector encoding the herpes simplex virus type 1 thymidine kinase gene and a hygromycin selection cassette (a gift of Drs. Jerry Shay and Woodring Wright, UT Southwestern Medical Center, Dallas, TX). HyTK cells were grown using KGM-2 serum-free culture media under hygromycin selection (EMD Biosciences, San Diego, CA).
Cytotoxicity Assay
To establish the efficacy of ganciclovir to induce a loss of cell viability in vitro, 5 × 105 HyTK cells were seeded onto collagen-coated glass coverslips in a 24 well culture plate (Thermo Fisher Scientific, Waltham, MA). An identical number of hTCEpi cells were plated in parallel as a control. Cells were grown at 37°C, 5% CO2 for 24 hours. After 24 hours, media was changed and cells were incubated in increasing concentrations of ganciclovir (0.1 – 10 μM) for 72 hours. Viability was assessed using a calcein-ethidium1 homodimer Live/Dead Viability Cytotoxicity Assay for mammalian cells (Invitrogen, Carlsbad, CA) by staining with 2 μM Calcein-AM and 4 μM Ethidium-1 in phosphate buffered saline (PBS, pH 7.2) for 45 minutes at room temperature. Cells were washed with PBS and mounted on slides using 50% v/v solution of glycerol-PBS. Cells were visualized using a fluorescent microscope (Leitz Diaplan, Wetzlar, Germany) equipped with a CoolSnap charge-coupled device camera (Photometrics, Tuscon, AZ). Viability counts were obtained from five respective visual fields using MetaMorph Software (Molecular Devices, Downington, PA). The experiment was repeated three times.
Epithelial removal and transplantation
A total of 52 athymic BALB/cAnNCr-nu/nu female mice (Charles River Laboratories, Voluntown, CT) were used in this study. Athymic mice were selected as they exhibit a deficient T-cell mediated-immune response and are commonly used for studies of epithelial transplantation.21, 22 All animals were at least 6 weeks old. Animals were treated according to the ARVO statement for the use of animals in ophthalmic and vision research. For removal of the existing epithelium, mice were anesthetized with an intraperitoneal injection of 100 mg/kg ketamine (Butler Animal Health Supply, Dublin, OH) and 10 mg/kg xylazine (Anased, Shenandoah, IA). One drop of topical 0.5% proparacaine (Bausch and Lomb, Rochester, NY) was instilled into each eye. Using round plastic tubing, proptosed eyes were exposed to 0.5 M EDTA for 20 minutes. Following EDTA treatment, a cellulose surgical spear (Braintree Scientific, Braintree, MA) was used to mechanically remove the epithelium. Eyes were subsequently treated with 0.25% mitomycin C (Sigma, St. Louis, MO) for an additional 20 minutes. This high concentration of Mitomycin C was necessary to effectively kill off any remaining epithelial cells and inhibit subsequent re-epithelialization of the cornea.23, 24 The concentration of mitomycin C was empirically determined (Table 1). After mitomycin C exposure, corneas were subsequently scraped to remove any residual epithelium and washed with PBS. For transplantation of HyTK cells to the corneal surface, a cell suspension of 1–15 × 106 cells in 200 μl of media was placed into the round plastic tubing for direct exposure to the denuded surface (Table 2). Cells were allowed to adhere for a minimum of 1 hour, while the mouse remained under anesthesia. The contralateral eye was used a control for epithelial re-growth.
Table 1.
Empirical determination of EDTA and Mitomycin C concentrations.
| Number of animals | EDTA | Epithelium removed |
|---|---|---|
| 9 | 2.5 mM | no |
| 4 | 0.25 M | no |
| 4 | 0.5 M | yes |
|
| ||
| Number of animals | Mitomycin C | Epithelial re-growth at 7 days |
|
| ||
| 3 | 0.02 % | yes |
| 5 | 0.1 % | yes |
| 3 | 0.25 % | none |
Table 2.
Visualization of one or more epithelial cell layers at 4 hours, 24 hours and 7 days. Cell number (×106) denotes number of cells per 200 μl in cell suspension used for epithelial transplant. For all animals reported, epithelium was not present in any control eye.
| Number of animals | Cell number (×106) | Presence of epithelium (number of animals with/total) |
|---|---|---|
| 4 hours | ||
| 3 | 5–10 | 3/3 |
| 24 hours | ||
| 5 | 1–4 | 0/5 |
| 8 | 5–10 | 7/8 |
| 3 | >10 | 3/3 |
| 7 days | ||
| 5 | ≥10 | 4/5 |
To confirm attachment of transplanted HyTK cells, cells were labeled pre-transplant with 5-chloromethylfluorescein diacetate (CellTracker Green CMFDA, Invitrogen, Carlsbad, CA), a membrane permeable cell tracker reagent. To label cells, cell pellets were incubated in 5 μM CMFDA for 45 minutes at 37°C. Staining solution was removed and cells were subsequently incubated in culture medium for 30 minutes. After an additional wash, cells were ready for direct transplantation. At 4 and 24 hours post-transplantation, animals were anesthetized with an intraperitoneal injection of 100 mg/kg ketamine and 10 mg/ml xylazine and euthanized by cervical dislocation. Enucleated eyes were snap frozen in liquid nitrogen and cryostat sectioned. Nuclei were counterstained using DRAQ5 (Alexis Biochemicals, San Diego, CA). All samples were mounted on slides using a 50% v/v solution of glycerol-PBS and imaged on a Leica SP2 laser scanning confocal microscope (Leica Microsystems, Heidelberg, Germany) using either a 20× or 63× objective. For all other experiments, animals were allowed to recover for up to 7 days and then further evaluated.
Immunofluorescence
For immunofluorescence studies, whole mouse globes were excised and fixed in RNase free 4% paraformaldehyde (Electron Microscopy Sciences, Fort Washington, PA) in PBS, embedded in tissue embedding medium (Leica Instruments GmbH, Nussloch, Germany) and snap frozen in liquid nitrogen. Cryostat-sectioned tissue was permeabilized with cold acetone (−20°C) and washed with PBS. Tissue was blocked with 10% donkey serum (Jackson ImmunoResearch, West Grove, PA) for 30 minutes at 37°C and incubated in a rabbit polyclonal anti-laminin (Sigma-Aldrich, St. Louis, MO) for 1 hour at room temperature or a mouse monoclonal anti-human keratin 3 (Serotec, Kidlington, Oxford, UK) overnight at 4°C. Samples were subsequently washed in PBS and stained with a FITC-conjugated secondary donkey antibody fragment (Jackson ImmunoResearch, West Grove, PA) for one hour at 37°C. Stratification was assessed using Alexa Fluor 488 Phalloidin (Invitrogen, Carlsbad, CA). Nuclei were counterstained with propidium iodide (PI, Sigma, St. Louis, MO). All samples were mounted on slides using a 50% v/v solution of glycerol-PBS and imaged on a laser scanning confocal microscope as described above.
Statistical Analysis
Statistical analysis was performed using SigmaStat 3.1 (Systat Software, Inc., San Jose, CA). All data are expressed in mean ± standard deviation. Normality and equal variance assumption testing were performed using the Kolmogorov-Smirnov Test and the Levene median test. For ganciclovir killing curve experiments, a two-way ANOVA with a Student Newman Khuels post hoc comparison test was used. Statistical significance was set at p<0.05.
Results
Epithelial debridement and seeding of HyTK cells on mouse cornea
Optimal concentrations of EDTA and Mitomycin C for use in epithelial removal were empirically determined (Table 1). Following debridement, examination of the resultant corneal surface using a laminin antibody demonstrated staining throughout the corneal surface in the denuded cornea, similar to that seen in the undisturbed eye, indicating that the basement membrane, at least in part, was not thoroughly damaged or removed during the procedure (Figures 1A & B). No staining was noted in the negative control (Figure 1C). To evaluate the capacity of a concentrated suspension of HyTK cells to adhere to the denuded corneal surface, HyTK cells labeled with CMFDA were placed directly on the proptosed eye and allowed to adhere for 1 hour. Examination at 4 hours demonstrated apparent attachment of HyTK cells to the cornea, with some cells appearing rounded and some cells demonstrating a more flattened morphology (Figures 2A–C). Examination of corneas at 24 hours post-seeding demonstrated a multi-layered appearance, with two layers apparent in some regions (Figures 2D–F, Table 2). In comparison to cells seen at 4 hours, at this later time point all cells had taken on a flattened, squamous morphology. Importantly, no corneal epithelial cells were noted in the non-seeded control eye at either time point examined.
Figure 1.
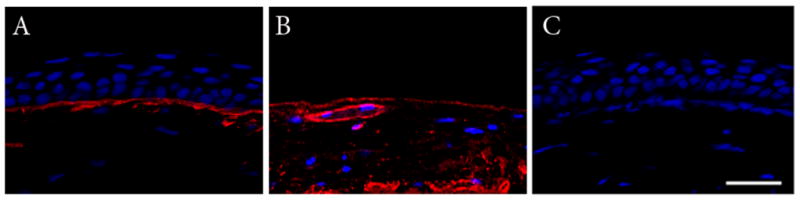
Double-labeling with anti-laminin (red) and DRAQ5 (blue). (A) Normal mouse cornea. (B) Presence of basement membrane following epithelial removal, note the presence of a blood vessel within the central cornea. (C) Negative control, primary antibody omitted. All images presented at identical magnification. Scale: 30 μm.
Figure 2.
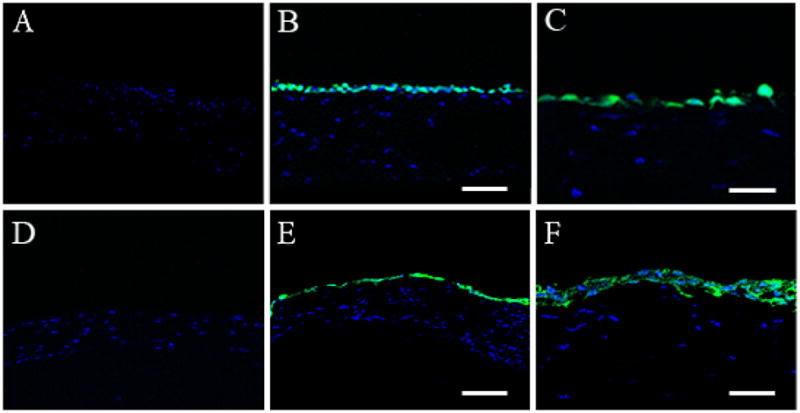
CMFDA CellTracker labeling of HyTK cells (green). DRAQ5 counterstaining of all nuclei (blue). (A–C) 4 hours post-transplantation. (A) Control eye confirming the absence of epithelium; (B) Labeled HyTK cells were seen evenly distributed throughout the corneal surface, scale: 80 μm; (C) Higher magnification showing an intermittent, single layer of HyTK cells, with occasional flattened cells, indicating that cells were adhering to the underlying basement membrane, scale: 40 μm. (D–F) 24 hours post-transplantation. (D) Control eye showing no evidence of re-epithelialization; (E) HyTK cells demonstrating increased flattening with concurrent multi-layering, scale: 80 μm; (F) Higher magnification confirming the presence of 2 cell layers, scale: 40 μm.
Stratification and differentiation of HyTK cells in vivo
To assess the ability of HyTK cells to stratify and differentiate into a fully reconstituted corneal epithelium, adherent HyTK cells were examined after 7 days of growth in vivo (Table 2). Phalloidin-FITC staining of cryostat sectioned tissue showed the presence of a five cell layered epithelium (Figure 3). Cells at the surface appeared squamous in nature, similar to native tissue. Basal cells were for the most part cuboidal in appearance, but appeared to lack the columnar morphology evident in the normal mouse cornea. A lower magnification montage of the cornea demonstrated that the multi-layered epithelium extended across the entire cornea; no epithelium was evident in the control eye (Figure 4). K3 staining confirmed differentiation into a corneal epithelial specific phenotype (Figure 5).
Figure 3.
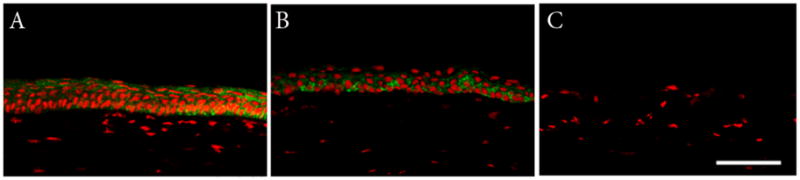
Double-labeling with Alexa Fluor 488 Phalloidin and PI. (A) Normal mouse corneal epithelium. Note the presence of 5–7 cell layers with columnar basal cells, wing cells, and flattened squamous surface cells. (B) HyTK cells grown for 7 days show 4–5 defined cell layers, with cuboidal basal cells and flattened squamous surface cells. (C) Negative control, primary antibody omitted. All images presented at identical magnification. Scale: 75 μm.
Figure 4.
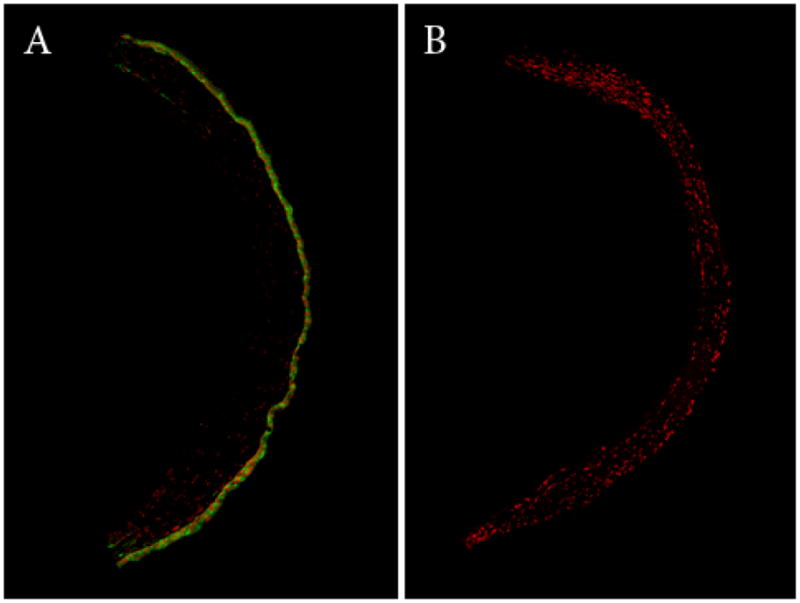
Montage of corneal surface double labeled with Alexa Fluor 488 Phalloidin and PI. (A) HyTK cells grown for 7 days; (B) Control eye, no evidence of re-epithelialization.
Figure 5.
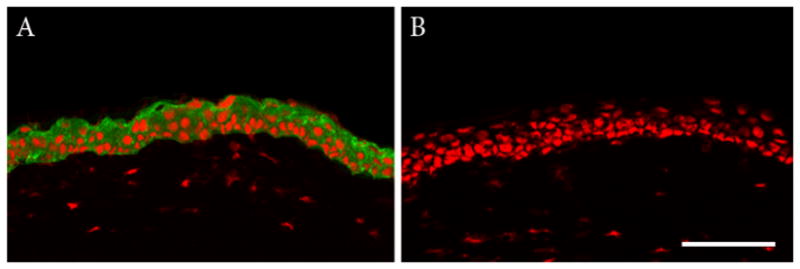
Double-labeling with cytokeratin K3 and PI. (A) After 7 days, the 4–5 cell layered reconstituted epithelium was positive for K3 in all basal and suprabasal cells. (B) Negative control, primary antibody omitted. All images presented at identical magnification. Scale: 75 μM.
Efficacy of ganciclovir for induction of cell death in HyTK cells in vitro
To evaluate the efficacy of ganciclovir in inducing cell death in the HyTK cell line, cells were seeded on collagen-coated coverslips and incubated in increasing concentrations of ganciclovir. Using live/dead assay, there was a significant decrease in cell viability with increasing concentrations of ganciclovir (Figure 6, p<0.001, n=3, two-way ANOVA, SNK post hoc multiple comparison test). The largest effect on viability was seen following treatment with 10 μM ganciclovir, where only 30% of remaining cells were viable. In comparison, hTCEpi cells not expressing the ganciclovir-TK vector were resistant to exposure to ganciclovir and no loss in cell viability was detected.
Figure 6.
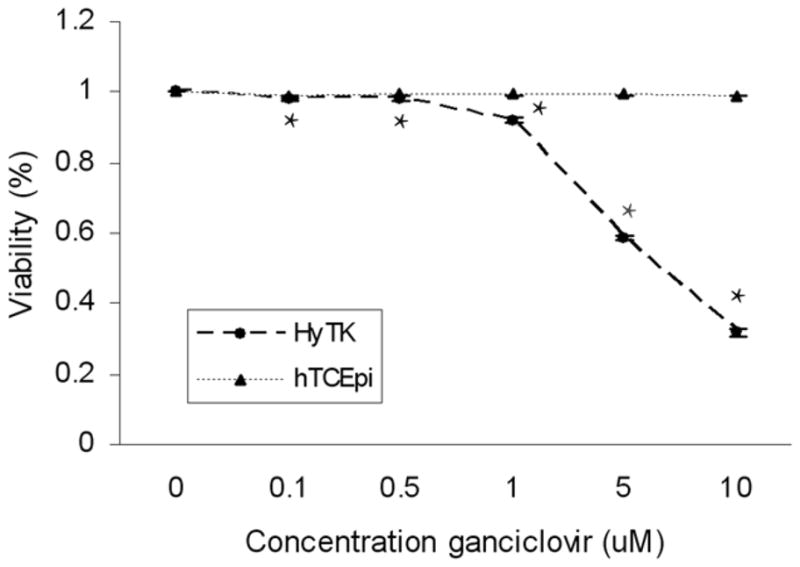
Ganciclovir cytotoxicity assay. HyTK cells incubated for 72 hours in increasing concentrations of ganciclovir showed a significant increase in cell death at all concentrations tested with only 30% cell viability at 10 μM (p<0.001, two-way ANOVA, SNK post hoc multiple comparison test, n=3). Graph representative of three combined experiments. No loss in viability was evident in the hTCEpi cell line.
Discussion
The results from this pilot study support the first in vivo demonstration of the use of a telomerase-immortalized corneal epithelial cell line to generate a full thickness corneal epithelium. Importantly, the use of a membrane permeable fluorescent cell tracker to label HyTK cells prior to transplantation confirmed that the newly developed HyTK cell line has the capacity to adhere to and multi-layer on the denuded stromal surface. These data further demonstrate that over time, these cells retain the potential to stratify into a multi-layered epithelium similar to the native cornea. Previous reports characterizing the differentiation potential of the hTCEpi cell line, from which the HyTK line was derived, demonstrated K3 expression after sequential submersed/air-lifted culture.12 The capacity to differentiate in vitro was further supported by the formation of ZO-1 mediated tight junctions along the apical surface, the production of MUC1 by apical cells, and the loss of nuclear BCL2 expression prior to TUNEL labeling, all indicating the formation of terminally differentiated surface cells.12 Further experiments are needed to fully characterize the differentiation profile of the HyTK cell line in vivo over longer times as well as to investigate the reestablishment of the limbal niche.
Removal of the existing epithelium proved to be a difficult task in the development of this model. Previous studies have reported the removal of the epithelium following the application of N-heptanol;25, 26 however, in the current mouse model we were unable to achieve the same effect. The use of mitomycin C at such a high concentration was effective in removing residual cells from the corneal surface as well as preventing re-epithelialization over the relatively short time course evaluated in this study. One of the primary drawbacks to utilizing such a high concentration however, is the corresponding loss of keratocytes.24, 27, 28 The degree of loss varied between mice, as some had significant loss throughout the anterior and posterior stroma, where others appeared to be restricted more to the anterior stroma. While there are obvious cellular signaling interactions between keratocytes and the epithelium, the significance of keratocyte loss on the long term viability and integrity of the epithelium is unknown. Likewise, in many of the 7 day mouse corneas, increases in the apparent keratocyte number were observed, suggesting that enough keratocytes remained viable following treatment to initiate repopulation.
The use of immortalized cell lines for reconstitution of in vivo tissue provides a useful model for experimental study of developmental and differentiation programs. The use of this cell line as a clinical therapeutic modality, if successful, would allow for an unlimited cell source for these procedures.13 The need for immunosuppressive therapy to circumvent tissue rejection and concerns pertaining to the use of viral vectors and constituent activation of cellular oncogenes (ie: SV40), which are commonly used to extend proliferation capability in vitro, severely limit this application.29, 30 The development of the HyTK cell line with an intrinsic kill switch should uncontrolled proliferation occur, represents a novel use of the currently available technology.17, 19 Safety limitations to this method exist however, in the potential for sub-optimal expression of thymidine kinase in vivo and leaky expression with a subsequent bystander effect resulting in toxic effects on neighboring cells.31 It is important to note, that in this study, the ability of this system to induce cell death was only investigated in proliferating cells in vitro. While treatment with ganciclovir appeared efficacious in selectively inducing cell death in HyTK cells compared to the hTCEpi control and appears to be a promising technique, further studies are needed to investigate whether topical or oral treatment with ganciclovir would be equally as effective in vivo in eradicating aberrantly proliferating cells.
The critical limiting factor in the clinical implementation of an immortalized cell line for tissue engineering purposes is immune rejection. Importantly, the technology that is required for manipulation of the cellular genome, ranging from selective gene knockdown to intricately controlled regulation of gene expression,32 is now readily available, creating new possibilities for the development of a cell line that can be effectively engineered to target key immune-regulatory mechanisms and potentially eliminate the subsequent need for systemic immunosuppression. The ability to manipulate an epithelial cell line in vitro to enhance intrinsic anti-inflammatory and anti-rejection signaling pathways may open a future avenue for therapeutic management of bilateral stem cell deficiency when currently available approaches have failed. In summary, the ability of the HyTK cell line to adhere and stratify to the damaged mouse cornea represents a novel model for studying differentiation and epithelial-niche interactions in vivo with potential applications in tissue engineering.
Acknowledgments
Supported in Part by NIH Grant R01 EY018219 (DMR), Core Grant EY020799, OneSight Research Foundation, Dallas, Texas (DMR), and a Career Development Award (DMR) and an unrestricted grant from Research to Prevent Blindness, Inc., New York, New York.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Cotsarelis G, Cheng SZ, Dong G, Sun TT, Lavker RM. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell. 1989;57:201–209. doi: 10.1016/0092-8674(89)90958-6. [DOI] [PubMed] [Google Scholar]
- 2.Lavker RM, Tseng SC, Sun TT. Corneal epithelial stem cells at the limbus: looking at some old problems from a new angle. Exp Eye Res. 2004;78:433–446. doi: 10.1016/j.exer.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 3.Lavker RM, Dong G, Cheng SZ, Kudoh K, Cotsarelis G, Sun TT. Relative proliferative rates of limbal and corneal epithelia. Implications of corneal epithelial migration, circadian rhythm, and suprabasally located DNA-synthesizing keratinocytes. Invest Ophthalmol Vis Sci. 1991;32:1864–1875. [PubMed] [Google Scholar]
- 4.Ladage PM, Yamamoto K, Ren DH, Li L, Jester JV, Petroll WM, Bergmanson JPG, Cavanagh HD. Proliferation Rate of Rabbit Corneal Epithelium during Overnight Rigid Contact Lens Wear. Invest Ophthalmol Vis Sci. 2001;42:2804–2812. [PubMed] [Google Scholar]
- 5.Yamamoto K, Ladage PM, Ren DH, Li L, Petroll WM, Jester JV, Cavanagh HD. Bcl-2 Expression in the human cornea. Exp Eye Res. 2001;73:247–255. doi: 10.1006/exer.2001.1027. [DOI] [PubMed] [Google Scholar]
- 6.Ren H, Wilson G. Apoptosis in the Corneal Epithelium. Invest Ophthalmol Vis Sci. 1996;37:1017–1025. [PubMed] [Google Scholar]
- 7.Araki-Sasaki K, Ohashi Y, Sasabe T, Hayashi L, Watanabe H, Tano Y, Handa H. An SV40-immortalized human corneal epithelial cell line and its characterization. Invest Ophthalmol Vis Sci. 1995;36:614–621. [PubMed] [Google Scholar]
- 8.Mohan RR, Possin DE, Mohan RR, Sinha S, Wilson SE. Development of genetically engineered tet HPV 16-E6/E7 transduced human corneal epithelial clones having tight regulation of proliferation and normal differentiation. Exp Eye Res. 2003;77:395–407. doi: 10.1016/s0014-4835(03)00175-1. [DOI] [PubMed] [Google Scholar]
- 9.Offord EA, Sharif NA, Mace K, Tromvoukis Y, Spillare EA, Avanti O, Howe WE, Pfeifer AMA. Immortalized human corneal epithelial cells for ocular toxicity and inflammation studies. Invest Ophthalmol Vis Sci. 1999;40:1091–1101. [PubMed] [Google Scholar]
- 10.Kahn CR, Young E, Lee IH, Rhim JS. Human Corneal Epithelial Primary Cultures and Cell Lines With Extended Life Span: In Vitro Model for Ocular Studies. Invest Ophthalmol Vis Sci. 1993;34:3429–3441. [PubMed] [Google Scholar]
- 11.Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S, Wright WE. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349–352. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- 12.Robertson DM, Li L, Fisher S, Pearce VP, Shay JW, Wright WE, Cavanagh HD, Jester JV. Characterization of Growth and Differentiation in a Telomerase-Immortalized Human Corneal Epithelial Cell Line. Invest Ophthalmol Vis Sci. 2005;46:470–478. doi: 10.1167/iovs.04-0528. [DOI] [PubMed] [Google Scholar]
- 13.Shay JW, Wright WE. The use of telomerized cells for tissue engineering. Nature Biotech. 2000;18:22–23. doi: 10.1038/71872. [DOI] [PubMed] [Google Scholar]
- 14.Simonsen JL, Rosada C, Serakinci N, Justesen J, Stenderup K, Rattan SIS, Jensen TG, Kassem M. Telomerase expression extends the proliferative life-span and maintains the osteogenic potential of human bone marrow stromal cells. Nature Biotech. 2002;20:592–596. doi: 10.1038/nbt0602-592. [DOI] [PubMed] [Google Scholar]
- 15.Jiang X-R, Jimenez G, Chang E, Frolkis M, Kusler B, Sage M, Beeche M, Bodnar AG, Wahl GM, Tlsty TD, Chiu C-P. Telomerase expression in human somatic cells does not induce changes associated iwth a transformed phenotype. Nature Genetics. 1999;21:111–114. doi: 10.1038/5056. [DOI] [PubMed] [Google Scholar]
- 16.Mondello C, Chiesa M, Rebuzzini P, Zongaro S, Verri A, Colombo T, Giulotto E, D'Incalci M, Franceschi C, Nuzzo F. Karyotype instability and anchorage-independent growth in telomerase-immortalized fibroblasts from two centenarian individuals. 2003;308 doi: 10.1016/s0006-291x(03)01484-0. [DOI] [PubMed] [Google Scholar]
- 17.Dachs GU, Tupper J, Tozer GM. From bench to bedside for gene-directed enzyme prodrug therapy of cancer. Anti-Cancer Drugs. 2005;16:349–359. doi: 10.1097/00001813-200504000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Knox RJ. Gene-directed enzyme prodrug therapy (GDEPT) - recognizing the present limitations of gene therapy for the treatment of cancer. Curr Opin Invest Drugs. 2001;2:835–838. [PubMed] [Google Scholar]
- 19.Greco O, Dachs GU. Gene directed enzyme/prodrug therapy of cancer: historical appraisal and future prospectives. J Cell Physiol. 2001;187:22–36. doi: 10.1002/1097-4652(2001)9999:9999<::AID-JCP1060>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 20.Denny WA. Prodrugs for gene-directed enzyme-prodrug therapy (suicide gene therapy) J Biomed Biotech. 2003;1:48–70. doi: 10.1155/S1110724303209098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenberg AS, Mizuochi T, Sharrow SO, Singer A. Phenotype, specificity, and function of T cell subsets and T cell interactions involved in skin allograft rejection. J Exp Med. 1987;165:1296–1315. doi: 10.1084/jem.165.5.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doran TI, Vidrich A, Sunk T-T. Intrinsic and extrinsic regulation of the differentiation of skin, corneal and esophageal epithelial cells. Cell. 1980;22:17–25. doi: 10.1016/0092-8674(80)90150-6. [DOI] [PubMed] [Google Scholar]
- 23.Rajan MS, O'Brart DPS, Patmore A, Marshall J. Cellular effects of mitomycin-C on human corneas after photorefractive keratectomy. J Cataract Refract Surg. 2006;32:1741–1747. doi: 10.1016/j.jcrs.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 24.Sadeghi HM, Seitz B, Hayashi S, LaBree L, McDonnell PJ. In vitro effects of Mitomycin-C on human keratocytes. J Refract Surg. 1998;14:534–540. doi: 10.3928/1081-597X-19980901-11. [DOI] [PubMed] [Google Scholar]
- 25.Cintron C, Hassinger L, Kublin CL, Friend J. A simple method for the removal of rabbit corneal epithelium utilizing n-heptanol. Ophthalmic Res. 1979;11:90–96. [Google Scholar]
- 26.Homma R, Yoshikawa H, Takeno M, Kurokawa MS, Masuda C, Takada E, Tsubota K, Ueno S, Suzuki N. Induction of epithelial progenitors in vitro from mouse embryonic stem cells and application for reconstruction of damaged cornea in mice. Invest Ophthalmol Vis Sci. 2004;45:4320–4326. doi: 10.1167/iovs.04-0044. [DOI] [PubMed] [Google Scholar]
- 27.Wu KY, Hong SJ, Huang HT, Lin CP, Chen CW. Toxic effects of mitomycin-C on cultured corneal keratocytes and endothelial cells. J Ocul Pharmacol Ther. 1999;15:401–411. doi: 10.1089/jop.1999.15.401. [DOI] [PubMed] [Google Scholar]
- 28.Song J-S, Kim J-H, Yang M, Sul D, Kim H-M. Mitomycin-C concentration in cornea and aqueous humor and apoptosis in the stroma after topical mitomycin-C application. Cornea. 2007;26:461–467. doi: 10.1097/ICO.0b013e318030d217. [DOI] [PubMed] [Google Scholar]
- 29.Ahuja D, Saenz-Robles MT, Pipas JM. SV40 large T antigen targets multiple cellular pathways to elicit cellular transformation. Oncogene. 2005;24:7729–7745. doi: 10.1038/sj.onc.1209046. [DOI] [PubMed] [Google Scholar]
- 30.Butel JS. Viral carcinogenesis: revelation of molecular mechanisms and etiology of human disease. Carcinogenesis. 2000;21:405–426. doi: 10.1093/carcin/21.3.405. [DOI] [PubMed] [Google Scholar]
- 31.Lo H-W, Day C-P, Hung M-C. Cancer-specific gene therapy. Advances in Genetics. 2005;54:235–255. doi: 10.1016/S0065-2660(05)54010-0. [DOI] [PubMed] [Google Scholar]
- 32.Varley AW, Munford RS. Physiologically responsive gene therapy. Mol Med Today. 1998:445–451. doi: 10.1016/s1357-4310(98)01333-1. [DOI] [PubMed] [Google Scholar]


