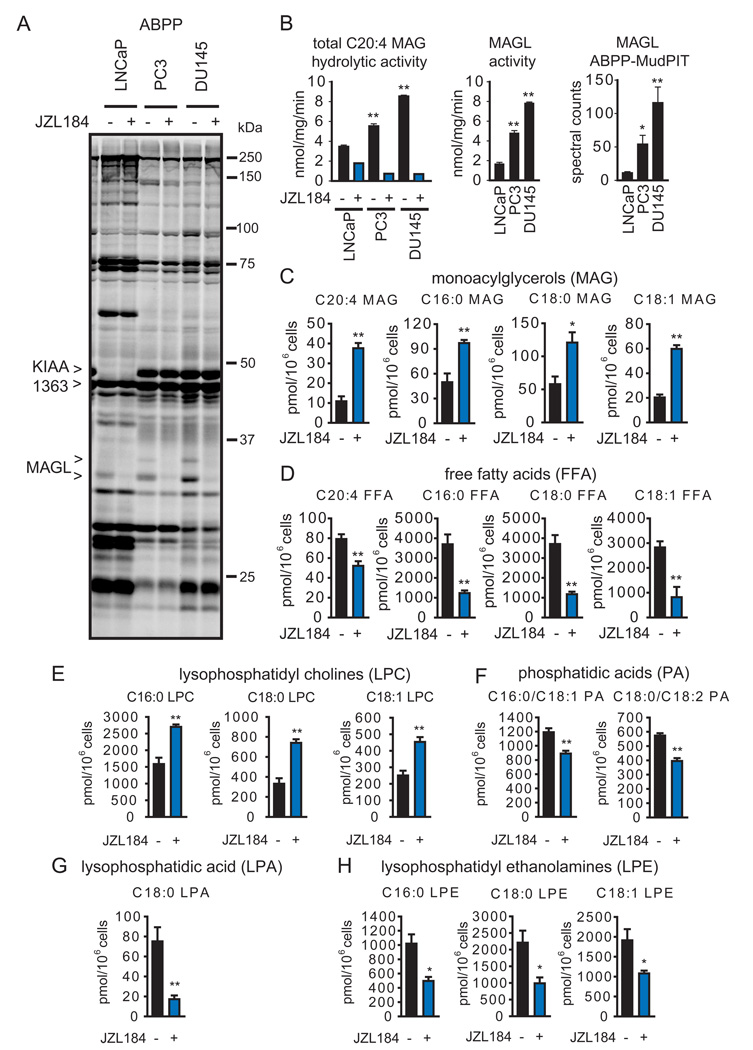
Figure 1. MAGL is elevated in androgen-independent prostate cancer cells where it regulates monoacylglycerol and free fatty acid metabolism.
(A) ABPP of serine hydrolase activities in the androgen-dependent LNCaP and androgen-independent PC3 and DU145 cells lines. Serine hydrolase activities were labeled in whole cell protoemes with the activity-based probe FP-rhodamine and detected by SDS-PAGE and in-gel fluorescence scanning (fluorescent gel shown in greyscale). MAGL and KIAA1363 are elevated in PC3 and DU145 cells compared to LNCaP cells. Proteomes were also prepared from cancer cells pretreated with DMSO or the selective MAGL inhibitor JZL184 (1 µM, 4 h in situ) to confirm the identities of the 33 and 35 kDa bands as MAGL. (B) The left panel shows C20:4 MAG hydrolytic activity of cancer cells in the presence or absence of JZL184 (1 µM, 4 h in situ). The middle panel shows MAGL-specific activity derived from subtracting the JZL184-insensitive portion from total MAG hydrolytic activity. The right panel shows spectral counts for MAGL in proteomes treated with FP-biotin and subjected to ABPP-MudPIT. (C,D) Inhibition of MAGL by JZL184 (1 µM, 4 h, in situ) raises MAG (C) and lowers FFA (D) levels in PC3 cells. (E–H) JZL184-treated PC3 cells also show elevations in lysophosphatidyl cholines (LPCs) (E), and reductions in phosphatidic acids (PAs) (F), lysophosphatidic acid (LPA) (G), and lysophosphatidyl ethanolamines (LPEs) (H). *p<0.05, **p<0.01 PC3 or DU145 versus LNCaP cells for (B) and JZL184-treated versus DMSO-treated control groups for (C, D). Data are presented as means ± standard error of the mean (SEM); n=4–5/group. See also Table S1, Table S2, and Figure S1.