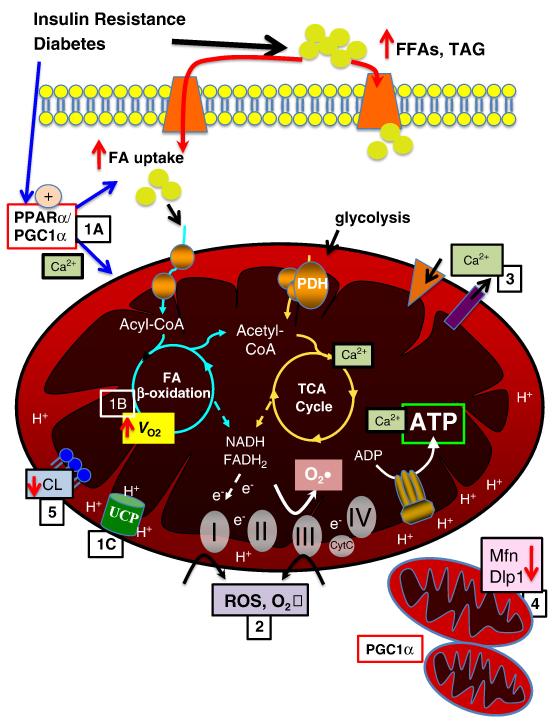
Figure 1. Mechanisms contributing to mitochondrial dysfunction.
Insulin resistance and diabetes lead to increased circulating free fatty acids (FFA) and triglyceride (TAG). There is increased fatty acid (FA) uptake via FA transporters (e.g. FATP, CD36), in part mediated by increased activity of PPARα and PGC-1α (1A). Increased FA uptake and PPARα contribute to increased FAO and oxygen consumption (VO2) (1B). In addition, proton transfer via uncoupling proteins (UCPs) contributes to uncoupling, reducing ATP production and contributing to cardiac inefficiency (1C). Increased FAO and oxidative phosphorlyation and impairment in the electron transport chain contribute to increased ROS and superoxide (O2•) production at complexes I and III (2), in addition a reduction in ROS scavenging enzymes may contribute to oxidative protein damage. Calcium transport across mitochondrial membranes is altered (3), impacting TCA cycle enzyme activity and ATP production. Emerging data suggest altere mitochondrial dynamics, with evidence of mitochondrial biogenesis, perhaps mediated in part by PGC-1α, as well as decreased levels of important regulators of mitochondrial fission, such as mitofusin 2 (Mfn) and dynamin-like protein (Dlp) (4). Mitochondrial membrane phospholipid content is altered, with evidence of decreased cardiolipin content, which may impact mitochondrial function via multiple modalities (5).