Figure 2. Assembly of PBA on a dimerizing scaffold.
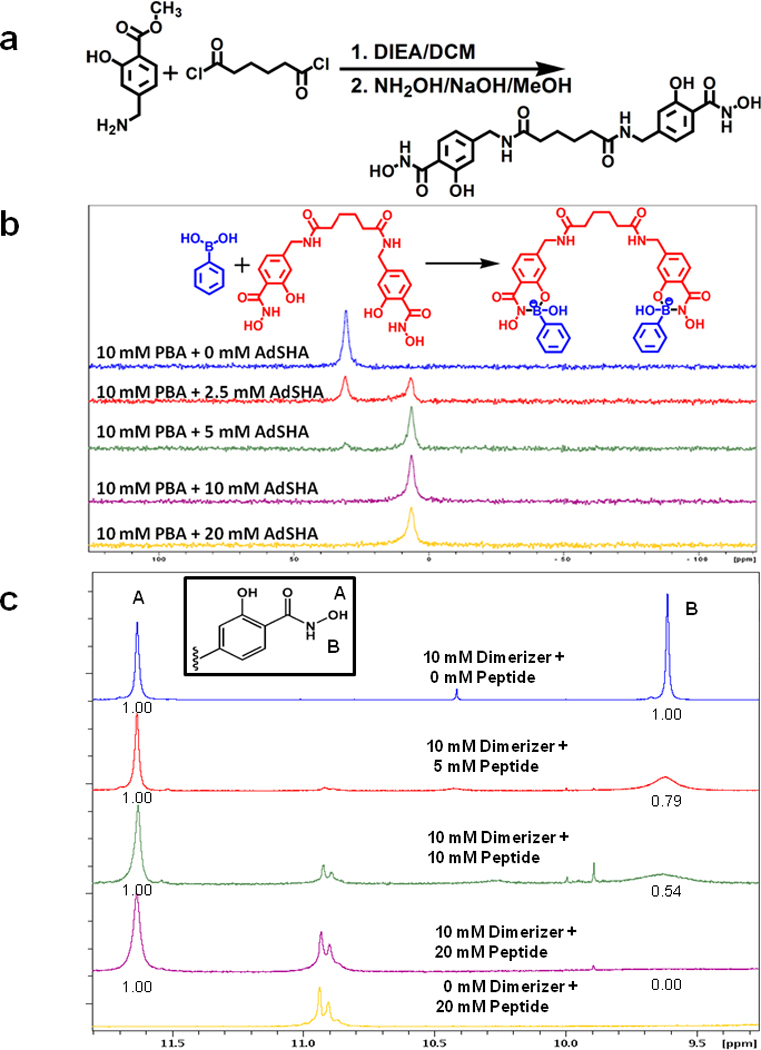
(a) Synthetic scheme of “dimerizer” synthesis.
(b) Titration of a dimerizer reveals stoichiometric complexation with PBA. 11B-NMR spectra of 10 mM PBA and 0 mM, 2.5 mM, 5 mM, 10 mM, and 20 mM of the dimerizer in 6% DMSO-d6/ 100 mM of deuterated phosphate buffer in D2O (pD = 7.4). PBA is largely complexed when present at a 2:1 ratio with dimerizer, as evidenced by the appearance of the tetrahedral coordination state of boron (6.7 ppm), and loss of the free PBA, which exhibits tetrahedral boron coordination geometry (30.8 ppm).
(c) 1H NMR spectra of dimerizer and PBA-modified c-Mpl-activating peptide at various relative concentrations in DMF-d7. The inset shows the structure of one of the two SHA present on the dimerizer. The hydroxamate O-H proton is labeled A and this proton does not participate in the complex formation. The hydroxamate N-H proton is labeled B and this proton is removed when the nitrogen atom forms a dative coordinate bond with boron in the peptide. The numbers below peaks A and B represent the relative area under each peak. With increasing concentrations of the peptide, the area under peak B is diminished, indicating the complex formation.
The peak assignments for protons A, and B were made based on the previous reports (Garcia et al., (2007).
