Figure 3. Synthesis of an active c-Mpl ligand in situ.
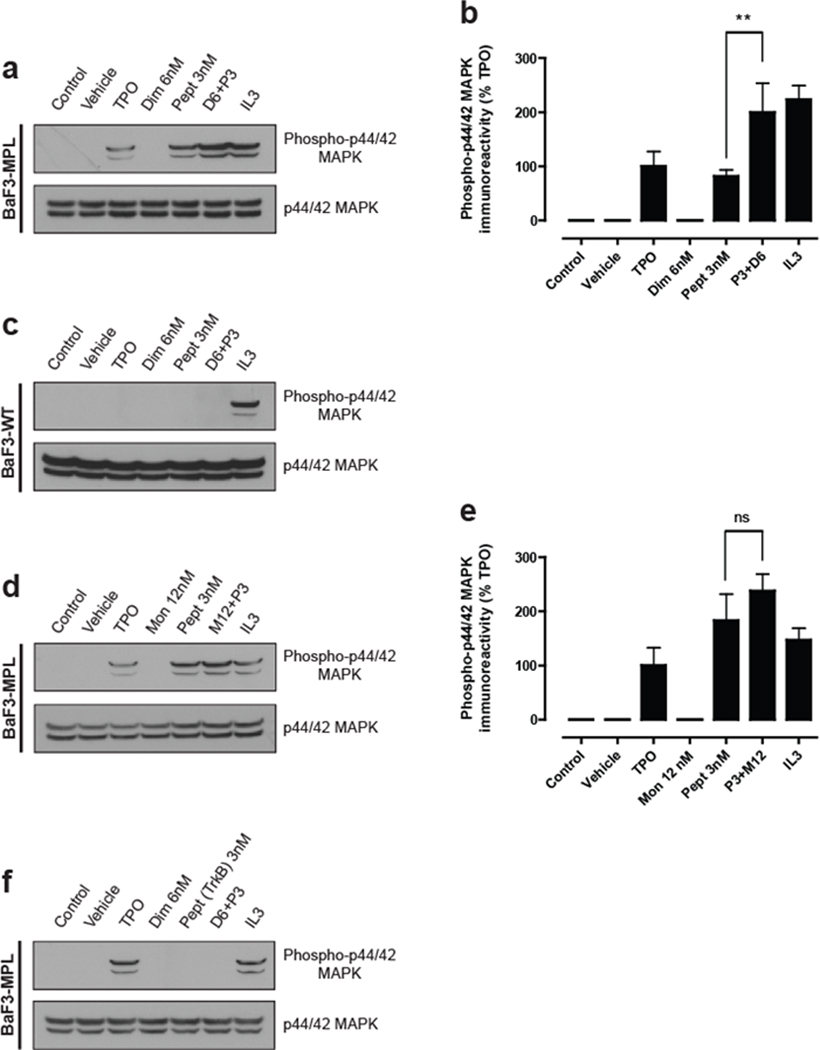
(a–f) BaF3-cMpl and BaF3-WT (parental) cells were transferred to media lacking IL-3, and cultured for 5 h. The cells were then stimulated with the indicated compounds for 10 min at 37°C and analyzed by immunoblot against phospho-p44/42 MAPK (upper panel) and total p44/42 MAPK (lower panel). In each experimental condition the immunoreactivity obtained was calculated as a percentage of the total signal obtained. Data are presented as mean ± s.e.m. of four independent experiments and each immunoblot is representative of four independent experiments.
(a) The agonist activity of the c-Mpl peptide is enhanced by the dimerizer. BaF3-cMpl cells were stimulated with TPO and IL-3, resulting in enhanced phospho-p44/42 MAPK levels as measured by Western blotting. The PBA-modified c-Mpl peptide (P) is a weak agonist of c-Mpl signaling, which is enhanced by treatment with the dimerizer (D). The dimerizer does not exert any agonist activity on its own.
(b) Quantification of results in (a). Groups were compared using a one-way ANOVA and Newman-Keuls post-test: **p<0.001 (n =4) of P alone relative to P + D. Data are presented as mean ± s.e.m. of four independent experiments.
(c) The effects of peptide-dimer complexes are mediated through c-Mpl. BaF3-WT cells, which are the parental line for the BaF3-cMpl cells, do not express c-Mpl. Under the same conditions as (a), the c-Mpl peptide and the dimer fail to activate c-Mpl signaling as assayed by phospho-p44/42 MAPK Western blotting.
(d) The c-Mpl-binding peptide is not significantly activated by a monomerizer. SHA, a monomeric control (M) to the dimerizer used in (a), was used to form complexes with the PBA-modified c-Mpl binding peptide. Treatment of cells with SHA along with the c-Mpl peptide failed to significantly increase phospho-p44/42 MAPK levels. These data support the idea that the dimerizer induces the formation of a dimeric complex which is the most efficacious ligand to activate c-Mpl signaling.
(e) Quantification of the results in (d). Groups were compared using a one-way ANOVA and Newman-Keuls post-test: n.s. = non significant (n = 4) of P alone relative to P + M. Data are presented as mean ± s.e.m. of four independent experiments.
(f) Activation of c-Mpl signaling requires a c-Mpl-binding peptide. When a PBA-modified peptide that targets TrkB was used, no phospho-44/42 MAPK activation was detected with either the peptide alone or in conjunction with the dimerizer. These results suggest that activation of c-Mpl signaling is mediated by a sequence-specific interaction with c-Mpl by a dimerized peptide.
