Figure 2.
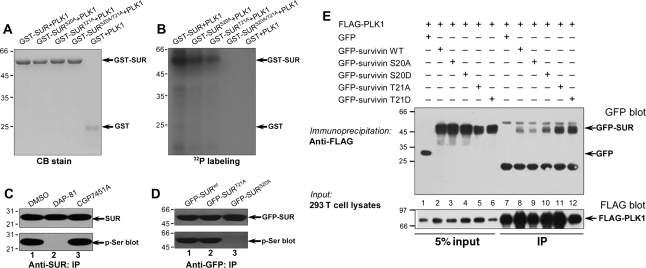
SUR is a substrate of PLK1 in dividing cells. Ser20 and Thr21 of SUR are substrates of PLK1. Bacterially recombinant GST–SUR fusion proteins were phosphorylated in vitro using [γ-32P]-ATP and active PLK1 as described under ‘Materials and methods’. Samples were separated by SDS–PAGE. (A) CB-stained gel of samples of wild type GST–SUR plus PLK1 (GST–SUR + PLK1), single and double mutant S20A/T21A GST–SUR plus PLK1 (GST–SURS20A/T21A + PLK1). (B) The same gel was dried and subsequently incubated with X-ray film. Note that there was dramatic incorporation of 32P into wild type, single site mutants (SURS20A and SURT21A) but little into the double mutant SURS20A/T21A or GST. (C) SUR is a cognate substrate of PLK1 in vivo. Extracts of nocodazole-synchronized mitotic HeLa cells were precipitated using an anti-SUR antibody. The precipitates were then fractionated by SDS–PAGE followed by immunoblotting of SUR (top panel) and serine phosphorylation (lower panel). Note that the SUR phosphorylation is a function of PLK1 but not CDK1. (D) Ser20 of SUR is phosphorylated in mitosis. GFP–SURwt, GFP–SURS20A and SURT21A were immunoprecipitated and isolated proteins were analyzed by western blotting. The membrane was first probed for GFP–SUR (upper panel) followed by detection of phosphoserine (lower panel). Lane 1, GFP–SURwt i.p.; lane 2, GFP–SURT21A i.p.; lane 3, GFP–SURS20A i.p. Note that GFP–SURS20A was not detected by phosphoserine antibody. (E) Association of PLK1 with SUR is independent of phosphorylation. Aliquots of HeLa cells were transiently transfected to express various GFP-tagged SUR plasmids with FLAG–PLK1. Thirty-six hours after transfection, these cells were lysed and clarified cell lysates were precipitated with FLAG-M2 agarose as described in ‘Material and methods’ section. Note that GFP–SURS20A weakened its association with PLK1.