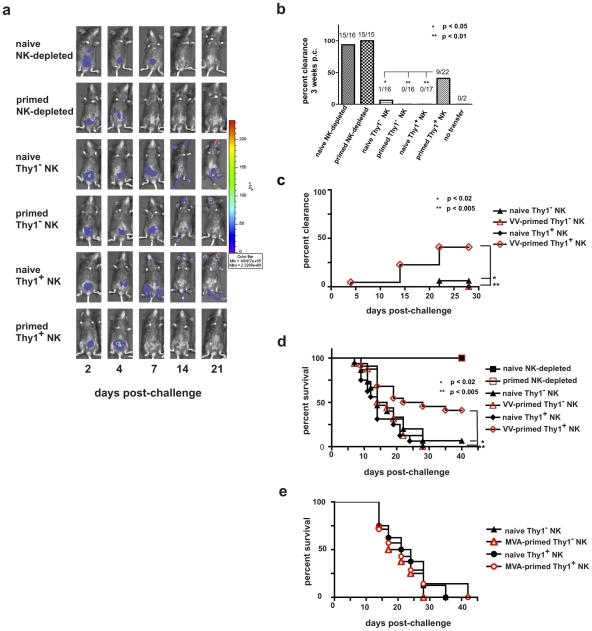
Figure 7. Innate immune protection against a vaccinia virus challenge is conferred on naïve RAG1ko mice by the adoptive transfer of primed Thy1+ liver-resident NK cells.
Six months after priming, 1×105 Thy1+ liver NK cells, 1×105 Thy1− liver NK cells, or 5×106 NK cell-depleted liver mononuclear cells from vaccinia virus-primed or naïve mice were adoptively transferred into RAG1ko mice. At the same time, mice were administered either control immunoglobulins (those mice that received NK-depleted cell transfers) or T cell-depleting monoclonal antibodies (those mice that received Thy1- and Thy1+ NK cell transfers). One week later the mice were challenged with 1×105 pfu rVV-luc intraperitoneally, and viral loads were monitored by IVIS imaging. (a) IVIS images of representative mice from each group over time following challenge are shown. (b) The ability of RAG1ko mice to resolve vaccinia virus infection by 3 weeks post-challenge is represented as the percent clearance within each group. Results were compiled from 4 separate experiments. RAG1ko mice that received primed Thy1+ liver NK cells showed enhanced clearance of vaccinia virus as compared to mice that received naïve Thy1+ NK cells, naïve Thy1-depleted NK cells, and primed Thy1-depleted NK cells (two-tailed Fischer test between groups). All statistics compare the test group relative to the group that received primed Thy1+ liver-resident NK cells. (c) The ability of RAG1ko mice to clear vaccinia virus infection throughout the course of challenge is represented as the percent clearance within each group. Results represent 4 separate experiments. RAG1ko mice that received primed Thy1+ liver NK cells showed enhanced protection against vaccinia virus challenge as compared to mice that received naïve Thy1+ NK cells, naïve Thy1− NK cells, and primed Thy1− NK cells. Statistical analysis was performed to compare Kaplan-Meier survival curves using GraphPad Prism 4 software. All statistics compare the test group relative to the group that received primed Thy1+ liver-resident NK cells. Protection provided by transferred primed Thy1+ hepatic NK cells surpassed the protection observed in mice that received hepatic naïve Thy1− NK cells (using the log rank test; p = 0.0168), primed Thy1− NK cells (p = 0.0043), and naïve Thy1+ NK (p = 0.0033). (d) Kaplan-Meier curves showing the survival of naïve RAG1ko recipients of the indicated adoptively transferred cell populations. Results represent the compilation of 4 separate experiments. RAG1ko mice that received primed Thy1+ liver NK cells showed enhanced survival of vaccinia virus challenge as compared to mice that received naïve Thy1+ NK cells, naïve Thy1− NK cells, or primed Thy1− NK cells. Statistical analysis was performed as in (c), demonstrating that host protection provided by transferred primed Thy1+ hepatic NK cells surpassed the protection observed in mice that received hepatic naïve Thy1− NK cells (log rank test; p = 0.013), primed Thy1− NK cells (p = 0.0049), or naïve Thy1+ NK (p = 0.0004). (e) Kaplan-Meier curves showing the survival of naïve RAG1ko recipients of the indicated adoptively transferred cell populations isolated from livers of naïve and MVA-primed mice. RAG1ko mice that received MVA-primed Thy1+ liver NK cells showed no evidence of enhanced survival following rVV-luc challenge as compared to mice that received naïve Thy1+ NK cells, naïve Thy1− NK cells, or MVA-primed Thy1− NK cells. Statistical analysis was performed as in (c and d). Results shown are representative of 2 independent experimental replicates.