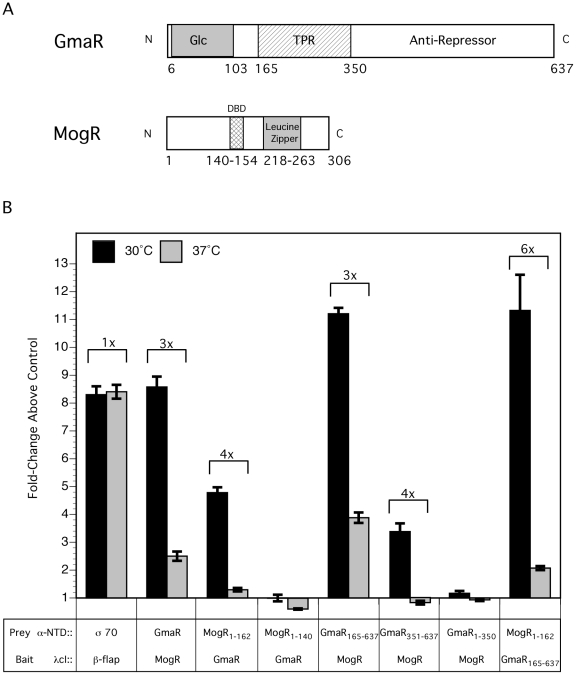
Figure 2. The interaction between GmaR and MogR is temperature-sensitive.
(A) Schematic representation of full-length GmaR and MogR proteins. The predicted glycosyltransferase (Glc) domain (amino acids 6–103) of GmaR is shaded in grey. Three tetratricopeptide repeat (TPR) domains (amino acids 165–350) of GmaR are represented as a hatched box. The MogR-binding anti-repressor domain of GmaR (amino acids 351–637) is labeled. The DNA binding domain (DBD) of MogR (amino acids 140–154) is indicated as a hatched box. The predicted leucine zipper domain (amino acids 218–263) of MogR is shaded in grey. (B) The E. coli two-hybrid assay results reported as fold-change above the negative control. Black bars represent the interaction at 30°C and grey bars 37°C. The difference in fold-change between the two temperatures is indicated above each interaction. Data represent the means and standard deviations of three independent experiments performed on the same day. Assays were performed on three separate days with similar results.