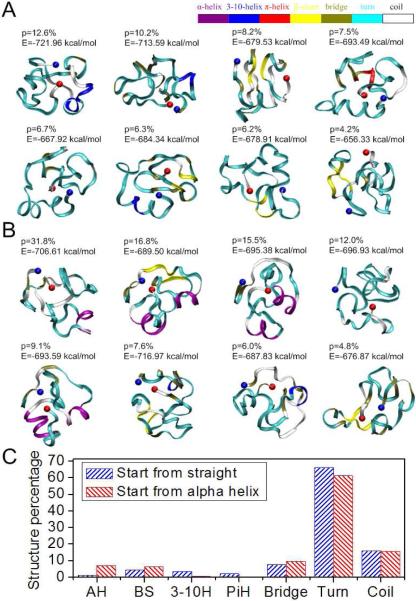
Figure 2. Conformations of the 50 amino acid segment of exon 11 with high significance factors.
Simulations of protein folding with initial conditions of (A) straight and (B) α-helix show similar final results, which are mostly disordered. Each amino acid is colored according to their secondary structure. The significance factor (p) as well as the potential energy (E) is marked for each conformation. The starting point (AA607) is in blue and the end point (AA656) is in red; (C) Secondary structure of the 50 amino acid segment shows little structure.