Table 1.
Enantioselective [3+2] Cycloadditions with a Heteroatom-Substituted Olefin: Effect of the Choice of Phosphine Catalysta
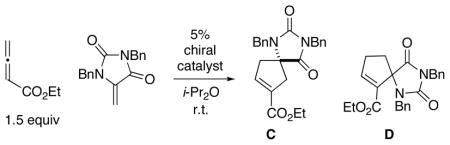 | ||||
|---|---|---|---|---|
| entry | chiral catalyst | ee (%)b | C: D | yield (%)c |
| 1 | (S,S)-DIPAMP | <2 | 1.0: 1 | 59 |
| 2 | (S,S)-Ph-BPE | <2 | 1.0: 1 | 66 |
| 3 | (S,S′, R,R′)-TangPhos | 11 | 0.8: 1 | 51 |
| 4 | (S)-2 | −51 | 0.8: 1 | 40 |
| 5 | (S)-3 | 59 | 1.1: 1 | 35 |
| 6 | (S)-4 | 76 | 1.2: 1 | 88 |
| 7 | (S)-1 | 97 | 1.3: 1 | 92 |
All data are the average of two experiments.
Enantiomeric excess of C. A negative value signifies that the R enantiomer was formed predominantly.
The yield was determined by HPLC analysis with the aid of an internal standard.
