Table 3.
Catalytic Enantioselective [3+2] Cycloadditions of γ-Substituted Allenes with a Phthalimide-Substituted Olefina
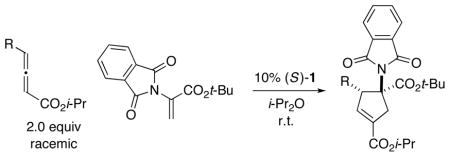 | |||
|---|---|---|---|
| entry | R | ee (%) | yield (%)b |
| 1 | Me | 98 | 81 |
| 2 |
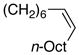
|
98 | 72 |
| 3 |
|
98 | 86 |
All data are the average of two experiments. For the determination of structure, including stereochemistry, see the Supporting Information.
Yield of purified product; for each cycloaddition, the ratio of regioisomers and diastereomers is ≥20:1 (determined by 1H NMR analysis of the unpurified reaction mixture).
